Vega Facility: GMP-Grade Commercial Manufacturing on Demand
Your Flexible CDMO Partner
At Exothera, we go beyond traditional CDMO services by offering flexible, on-demand GMP-grade space to support your manufacturing needs. Whether you need (Grade C) clean rooms, lab space, or customized capacity solutions, our Vega Facility in Jumet provides the perfect environment for viral vector and RNA therapeutic production.
Furthermore, we can design facilities and tech transfer those designs and operational plans to our clients.
📍 Location:
Zoning de Jumet, Allée Centrale 52,
6040 Jumet, Belgium
📞 Tel: +32 (0) 71 49 67 22
Facility Overview
Suite details
- 2x Grade C Units, each with:
- One Upstream Processing (USP) suite
- One Downstream Processing (DSP) suite
- Each suite: ~50m² (~550 sq. ft.)
- Each unit: ~100m² (~1,100 sq. ft.)
Key Facility Features
- Uninterrupted Power Supply & Backup Generator
- Buffer/Media Prep Area: 650L per batch
- Pre-Culture Suite
- On-Site Water Purification
- O₂, CO₂, N₂, and Compressed Air Supply (Additional gases available upon request)
- Segregated Office Space
Available equipment
Suspension culture
- 2x 200L Pall Allegro STR
- 2x 2,000L Pall Allegro STR
- 2x Sartorius RM50 Rocker
- 2x Sartorius RM200 Rocker
Adherent culture
- Scale-X Hydro (2.4m2)
- Scale-x Carbo (10m2 and 30m2)
- Scale-X Nitro (200m2 and 600m2)
Purification/Other capabilities
- Millipore Depth Filtration
- Akta Ready chromatography
- Akta Readyflux diafiltration
- Various mixers
Ancillary services
- Suite setup, monitoring (EM, IQOQ, EMPQ, etc.) and maintenance (cleaning and on-site utility technicians)
- Material qualification and DP release
- Personnel training and available operators
- Fill/finish on-site (manual)
- Warehouse, cold-storage
- Quality system management (including vendor qualification and material sourcing)
GMP-Grade Space for Collaboration
At Exothera, we believe in true partnership. Our clients get more than just clean rooms; they gain access to a flexible collaborative model, where they can integrate their employees into our teams or secure dedicated capacity for manufacturing surges.

Custom Development & Manufacturing Solutions
ISO-Certified GMP Clean Rooms
- Designed for commercial-scale manufacturing of viral vectors and RNA therapeutics.
- Flexible configurations for suspension and adherent cell cultures.
- Suited for in vitro transcription (IVT) of RNA.
- Cost-effective: No major capital investment required.
- Ready for use: GMP-qualified and available for immediate occupancy.
- Scalable: Adaptable configurations for different manufacturing needs.
- Versatile: Suitable for viral vectors, mRNA, antibodies, and more.
- Expert Support: On-site GMP professionals to assist with operations.
Flexible Clean Room & Capacity Solutions
We provide a range of solutions tailored to your production needs:
Turnkey clean room

- Fully equipped, ready-to-rent GMP space
- Configurable for suspension or adherent cell culture
- Size: 145m² (1,453 sq. ft.)
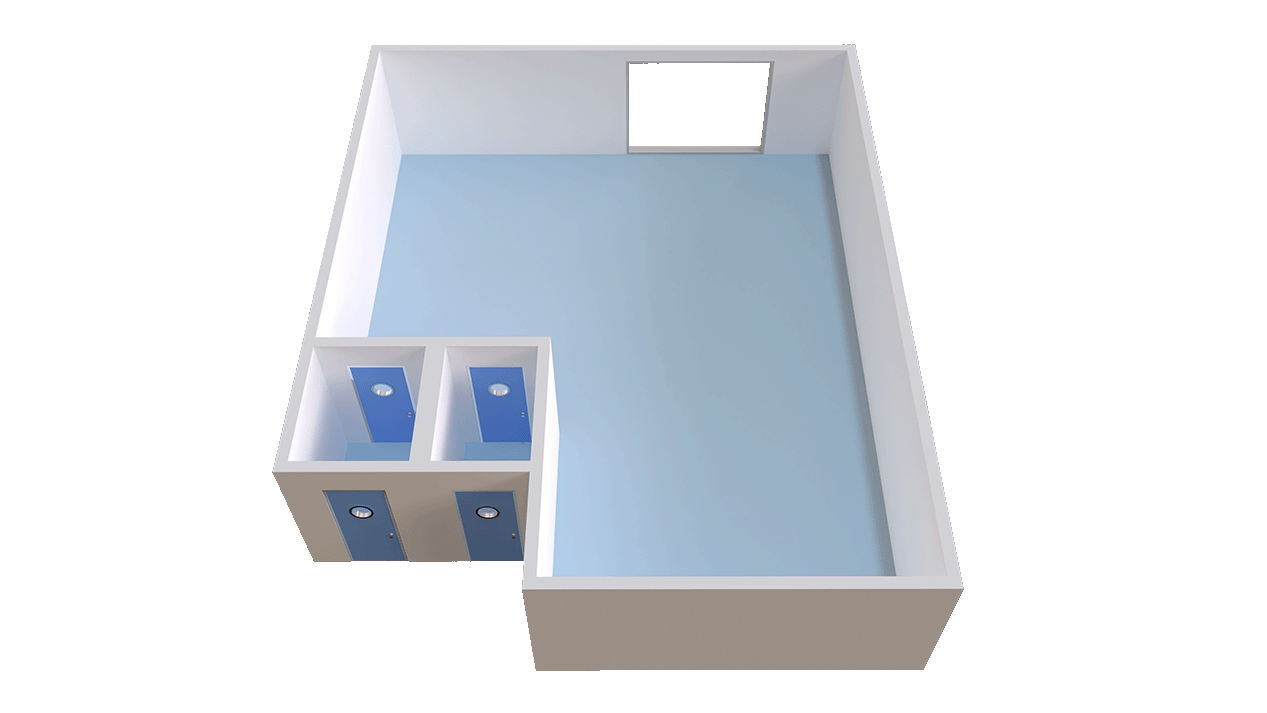
Ball room
-
Open, flexible space for GMP activities
-
Customizable for different manufacturing needs
-
Size: 92m² (990 sq. ft.)
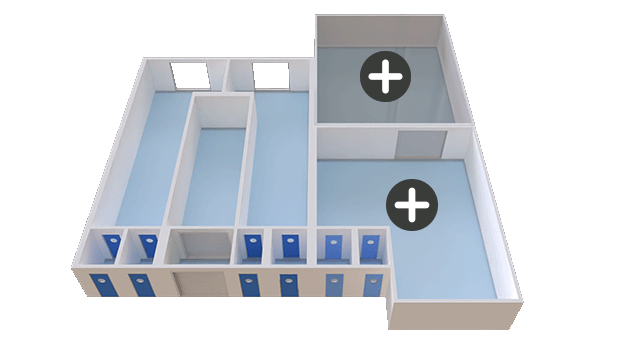
Capacity development
-
Custom-built capacity solutions tailored to your needs
-
Scalable and adaptable for long-term production
-
Size: Variable, based on requirements
Let’s Discuss Your Needs
Looking for the ideal GMP space for your next project? Our team is ready to help you find the perfect solution for your manufacturing and development goals.
Contact us today to explore your options!
