Accelerate Your Viral Vector Manufacturing
A Complete, Ready-to-Use Viral Vector Platform
The exoREADY™ viral vector platform integrates high-performance starting materials—including proprietary cell lines and vector systems—with our data-driven expertise and comprehensive CMC documentation package. This package includes standard operating procedures, testing specifications, and material guidelines. Our flexible manufacturing technology, combined with regulatory support, offers a seamless solution for viral vector-based vaccines, oncolytic therapies, and gene therapy products.

Why Choose exoREADY?
Optimized for Industrial Manufacturing
- ETailored for large-scale Cell and Gene Therapy (CGT) and Vaccine production.
Fully Customizable
- EAdaptable to specific process requirements, ensuring flexibility and efficiency.
Regulatory & CMC Support
- EStreamlined compliance with industry standards for a faster clinical transition.
Fast-Track Your Development Without Compromising Quality
Our mission is to reduce development timelines while maintaining the highest quality and cost efficiency.

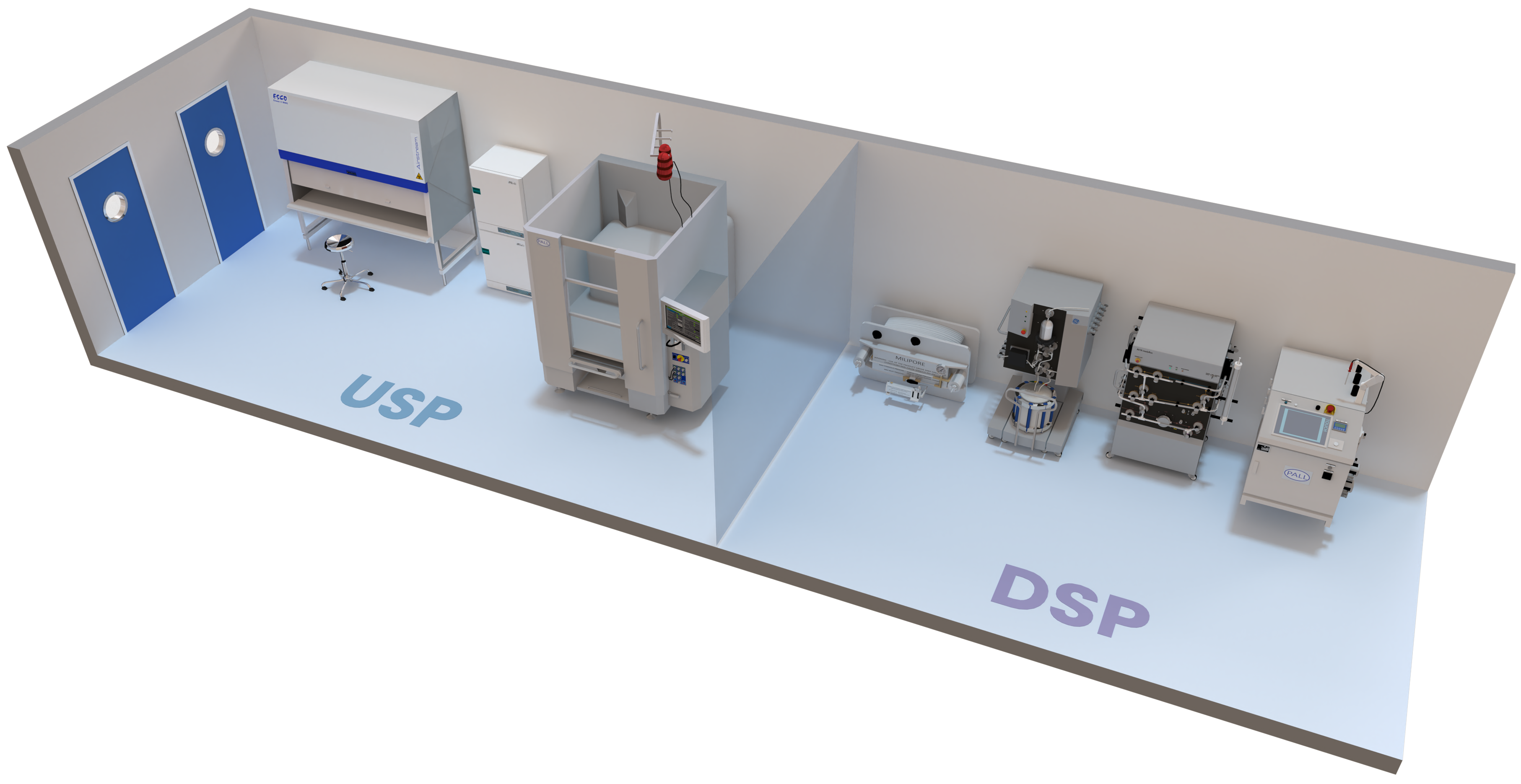
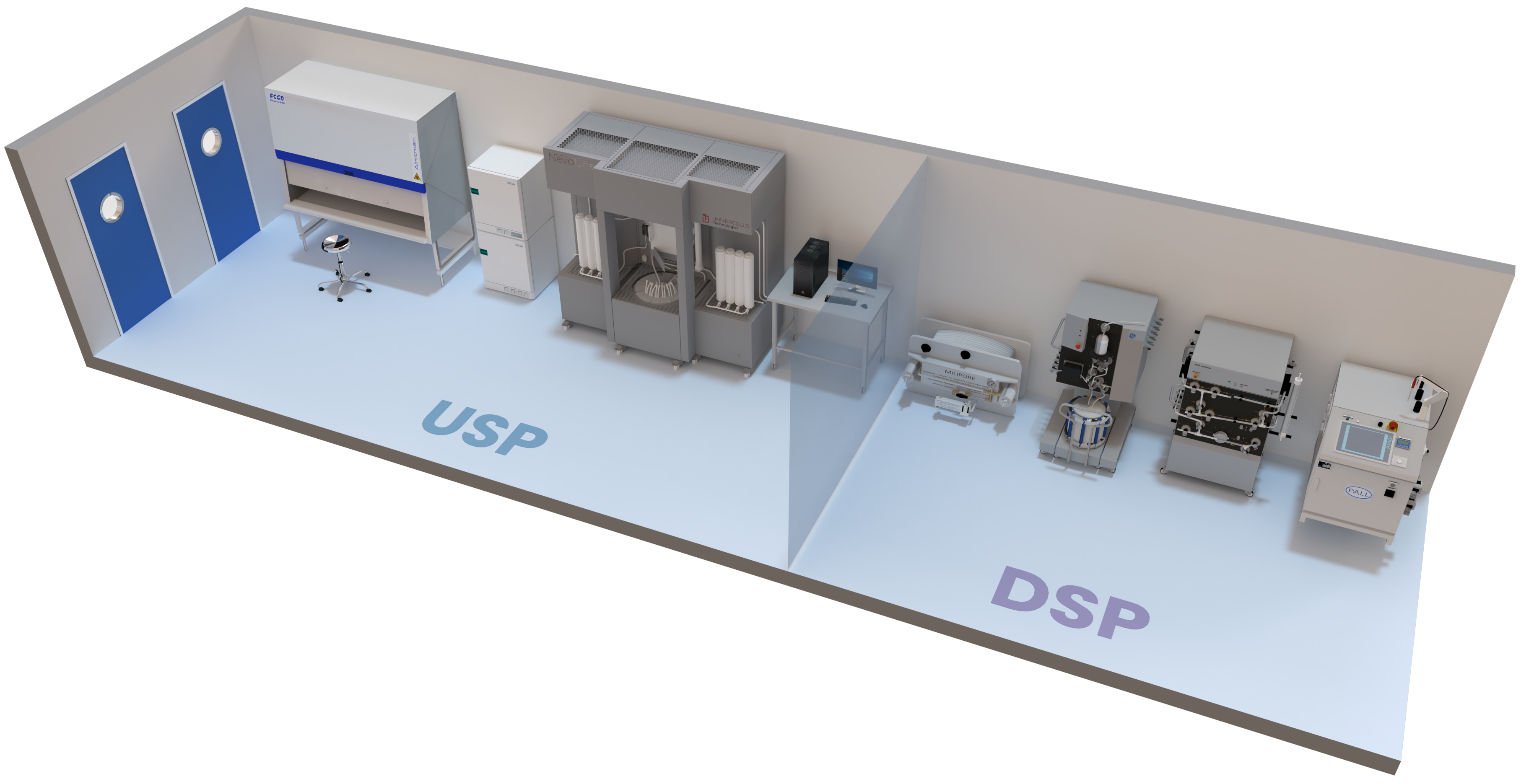
Advanced Manufacturing Processes
Developed for both adherent and suspension cell culture and equipped with the latest DSP technologies.
Unlock the Power of exoREADY™
Take advantage of our cutting-edge viral vector manufacturing technology to accelerate your pathway to clinical trials.