Fast-track your mRNA Therapy or Vaccine, without Compromising Quality
Unleash the innovative potential of Ntensify™, our advanced mRNA production platform. With the expertise of our dedicated RNA scientists, Exothera offers comprehensive mRNA development and manufacturing services. From early R&D to commercial GMP, including LNP encapsulation, we are your trusted partner in advancing your mRNA technology.
Exothera understands the unique challenges of mRNA drug development and offers tailored solutions to empower your breakthrough
Scalability
The Ntensify™ platform offers a streamlined approach to accelerate your mRNA-based product development. Our automated and optimized process scales linearly from R&D to clinical and commercial scales, eliminating the need for additional process development.
Time and Costs
Traditional IVT approaches require time-consuming and cost-prohibitive scale-up process development as you move from R&D to the clinic to commercial. The Ntensify™ platform is an automated, optimized process that eliminates the need for process development – fast-tracking your mRNA product to the clinic at a fraction of the cost.
Product quality
The Ntensify™ platform has been optimized to reduce process- and product-related impurities, consistently yielding high-quality mRNA. Combined with our robust suite of in-house QC analytics, you can trust Exothera to provide reliably high-quality mRNA at every stage of development.
Flexibility
We provide access to multiple capping technologies and LNP solutions along with your choice of uridine modifications to enhance the efficacy and safety of your mRNA drug product.
Regulatory Compliance
We offer CMC consultancy services to guide you through the regulatory process for your mRNA program. Our expertise helps streamline your regulatory submissions and increases the likelihood of successful approvals for your mRNA-based therapeutics or vaccines.
Simplified Process Transfer
The Ntensify™ platform is sequence-agnostic and compatible with various capping technologies, simplifying the adaptation of your current process. This allows seamless integration of your existing approach into our optimized platform, potentially boosting efficiency and enhancing product quality, regardless of your product’s development stage.
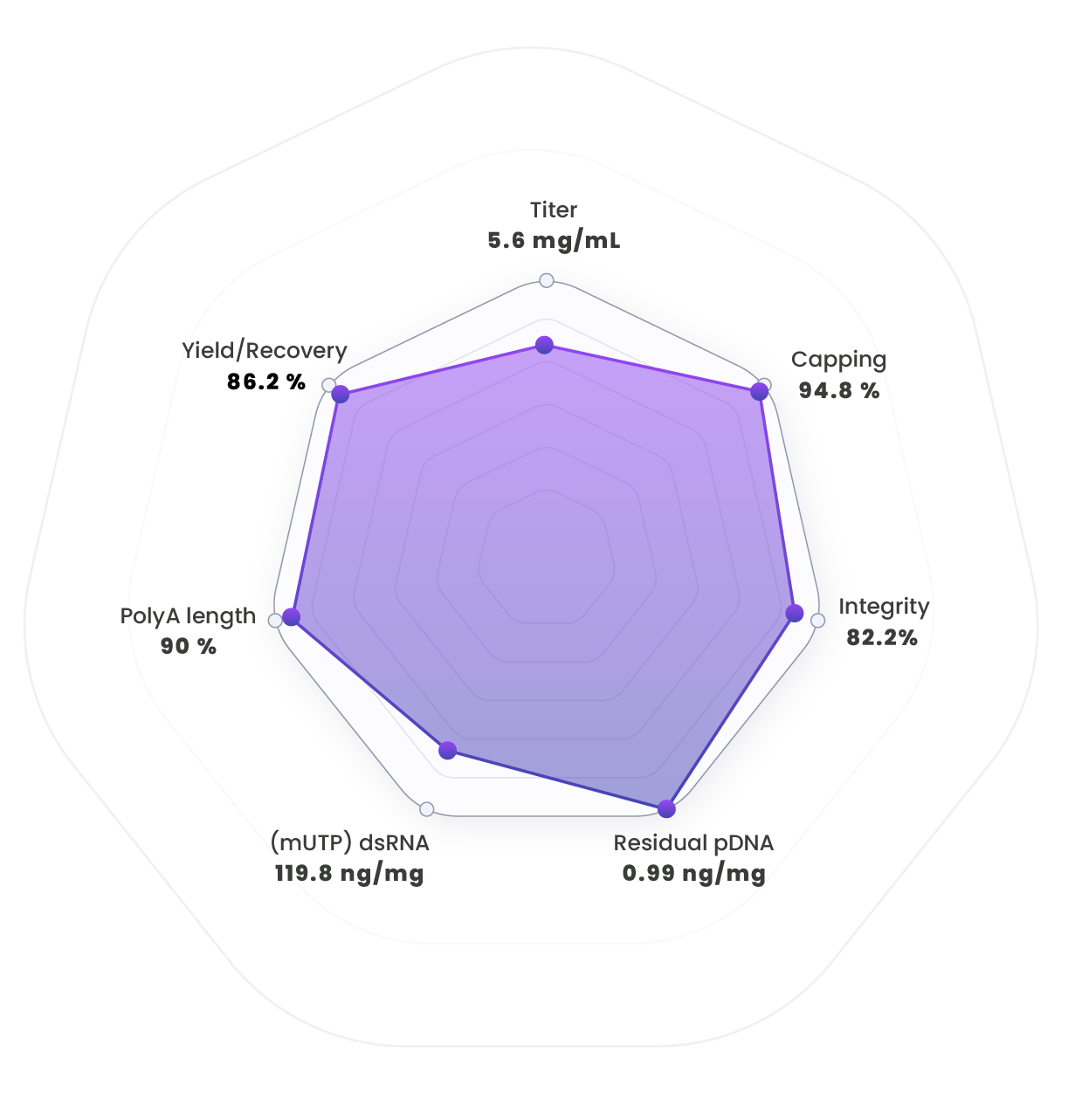
Average performances of the Ntensify® process related to process parameters and mRNA quality attributes.
RUO mRNA
• Reporter Genes
• Custom constructs with target yields starting at 200µg
Clinical Development
• Codon Optimization
• CMC consultancy for regulatory
• GMP-like production for tox screens and IND studies
Commercial Manufacturing
• Produce up to 5g purified RNA/day (up to 500k doses)
• LNP Encapsulation
• Fill and Finish Capabilities
Our analytical portfolio includes:
In-house DS
pH, Endotoxin, Bioburden, Appearance: Compendial
Residual DNA template: ddPCR
Residual total protein: uBCA
DNA concentration & purity: Nanodrop
Residual unincorporated nucleotides: HPLC
Linearization profile: Gel electro
mRNA sequencing & Poly a tail: Nanopore
mRNA integrity: Capillary electro
% of linearized DNA: Capillary electro
Identity by size: Starting mat.
Capping efficiency: UPLC
dsRNA: ELISA
In-house DP
Encapsulation yield: Fluorescence
Lipid content: HPLC-CAD
LNP size and polydispersity: DLS
In vitro Expression Assay: HEK293 transfection-ELISA
Endotoxins: LAL
Osmolality: Freezing-point depression
pH: Potentiometry
Residual solvents: Characterization
Sterility: Modified USP<71>
Outsourced
Residual Ethanol by GC-MS: Fluorescence
BioBurden: USP<61>
Any Questions For Us?
Our Locations
In Belgium, our state-of-the-art facilities, called VEGA and NOVA, are based on the Univercells Campus, in Jumet (Belgium), close to the Brussels South Airport. They offer 900 m² (9,687 ft²) of GMP production area for viral vectors and 500 m² (5,381 ft²) of ready-to-use production area for nucleic acids.
In North America, our 800m² (8611 ft²). Research Center of Excellence for RUO RNA manufacturing is located in Andover, Massachusetts.
Speak to our BDs:
Meet our Team:

Adam Thakore
Business Development Manager for Western EU, UK and PacAsia

Oliver Dezutter
Business Development Manager for Eastern Europe

Ana Martini
Business Development Director for Western North America

Manuel Valdes
Senior Director BD for Eastern North America

Andrew Klauk
Senior Inside Sales - Worldwide