
Continuous-batch RNA manufacturing platform – Get faster to tox without compromising on quality.
Ntensify™: Standardized & Scalable mRNA Manufacturing
A Ready-to-Use Platform for Seamless mRNA Production
The Ntensify™ platform, powered by Quantoom Biosciences, offers a fully standardized and optimized process across all production scales. Unlike conventional mRNA manufacturing that requires complex process development, Ntensify provides an off-the-shelf solution, eliminating variability and ensuring smooth scalability from R&D to GMP production.

Why choose Ntensify? No Process Development, Full GMP Compatibility
Traditional mRNA manufacturing demands extensive process development, often requiring a mix of raw materials that may not even be GMP-compliant. This leads to long lead times or even situations where obtaining GMP-grade materials is impossible. At Exothera, we solve this challenge by providing a fully standardized, off-the-shelf platform, including ready-to-use reagent mixes.
No Process Development Required
- EReady-to-use platform optimized from research to GMP
Consistent materials
- EStandardized high-quality reagent mixes, used at every scale, ensure quality and regulatory adherence
Flexibility
- EPossibility to be GMP-ready from 20mL – A crucial advantage for personalized cancer vaccines and other specialized therapies
Regulatory & CMC Support
- EDesigned to meet regulatory requirements with full documentation and compliance assistance
Breakthrough in mRNA Purification
A key differentiator of Ntensify is its one-step purification process, achieved using silica beads instead of oligo(dT). This unique approach streamlines purification, enhancing efficiency and reducing process complexity – a major advancement in the mRNA industry.

Scalability Without Compromise
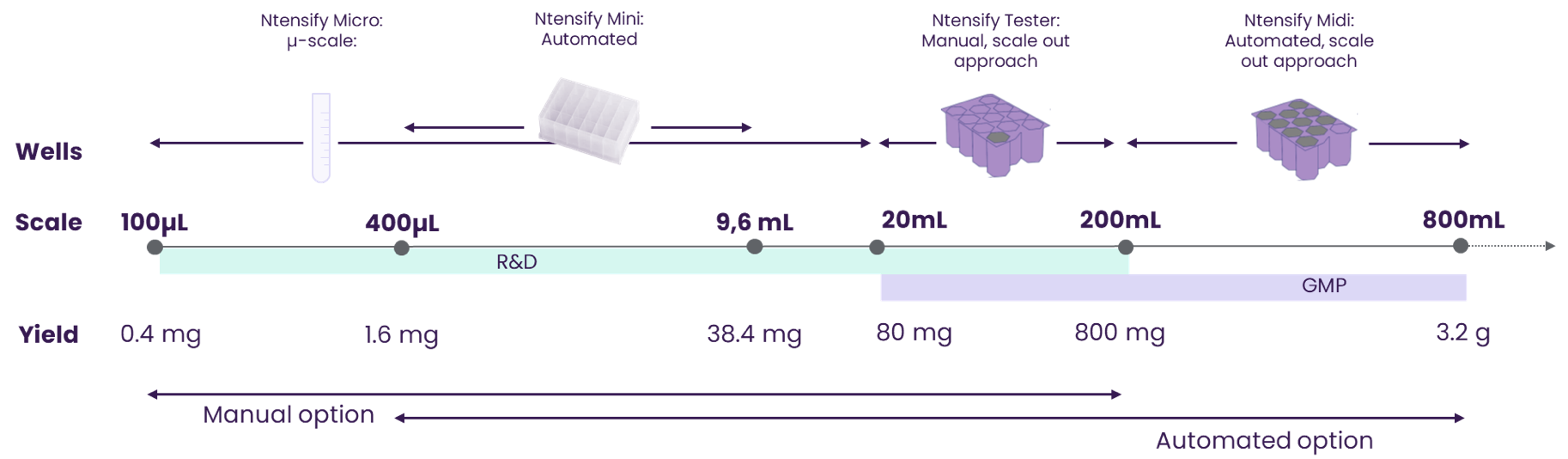
Scale OUT, Not Just Up
Our scalability is unmatched. Unlike traditional platforms that scale up, our system is designed to scale out, ensuring consistent results at every volume. Whether for personalized medicine or large-batch manufacturing, our system delivers unparalleled flexibility.
-
-
- R&D Scale: 50µL to 200mL per batch
- GMP Scale: 20mL to 800mL per batch
- Process Consistency: Reliable and reproducible outcomes at all stages
-
With in-house production of fully released, qualified, and optimized premixes, Exothera is setting a new industry standard for mRNA manufacturing.
Unlock the Power of Ntensify™
Join us in redefining the future of mRNA manufacturing. Our platform is designed for speed, flexibility, and GMP compliance, supporting innovations in personalized medicine, vaccines, and beyond.
Connect with our Business Development Team today and discover how Ntensify™ can accelerate your next breakthrough!