RNA MANUFACTURE FOR PRECLINICAL STUDIES
A preclinical package design that can reach commercial manufacturing without scale-up
Maintaining consistent process & increasing product quality for your RNA-based therapeutics
Our approach and expertise
Exothera works with you to manufacture your therapeutic for preclinical use through our ExoREADY platform which includes proprietary production processes and best-in-class equipment that can be applied to single constructs or multiple constructs per lot.
Through affordable and rapid proof of concepts, we can produce linear DNA stocks, purified mRNA, and encapsulated mRNA in non-GMP conditions:
- for material used in 1st phase screening studies (based on in vitro expression tests)
- for material used in 2nd phase screening studies (based on in vivo tests)
- for the production of toxicology lots when non-GMP conditions are required

Fast and optimized process for both DNA synthesis and RNA production
Exothera ensures secure access to DNA, thanks to our DNA service for screening purposes. Our optimal ExoREADY process ensures high quality at high speed, both during DNA synthesis as well as RNA production.
Starting from a cell-free working bank, we only need a few days to obtain the linear DNA template for mRNA production without fermentation, or plasmid linearization.
Thanks to our optimized process, we can generate the final DNA product ranging from 2 to 12kb with poly-A/T tail sizes from 40 to 120nt.
A single DNA template can then be transcribed to produce over 100 mg of the same RNA. Our ready-to-use process furthermore uses an optimized in-vitro transcription method which obviates the need for downstream dsRNA clearance while improving reaction yield and reducing consumption of capping reagent, all of this allowing for a faster speed to clinic with manufacturability and a significant reduction in CoGs.
The below data demonstrates that our ExoREADY process delivers consistently excellent yield, integrity and capping efficiency, together with very low levels of dsRNA and residual DNA.
Our approach and expertise
Through affordable and rapid proof of concepts, we can produce linear DNA stocks, purified mRNA, and encapsulated mRNA in non-GMP conditions:
- for material used in 1st phase screening studies (based on in vitro expression tests)
- for material used in 2nd phase screening studies (based on in vivo tests)
- for the production of toxicology lots when non-GMP conditions are required

Fast and optimized process for both DNA synthesis and RNA production
Starting from a cell-free working bank, we only need a few days to obtain the linear DNA template for mRNA production without fermentation, nor plasmid linearization.
Thanks to our optimized process, we can generate the final DNA product ranging from 2 to 12kb with poly-A/T tail sizes from 40 to 120nt.
A single DNA template can then be transcribed to produce over 100 mg of the same RNA. Our ready-to-use process furthermore uses an optimized in-vitro transcription method which obviates the need for downstream dsRNA clearance while improving reaction yield and reducing consumption of capping reagent, all of this allowing for a faster speed to clinic with manufacturability and CoGs in mind.
The below data demonstrates that our ExoREADY process delivers consistently excellent yield, integrity and capping efficiency, together with very low levels of dsRNA and residual DNA.
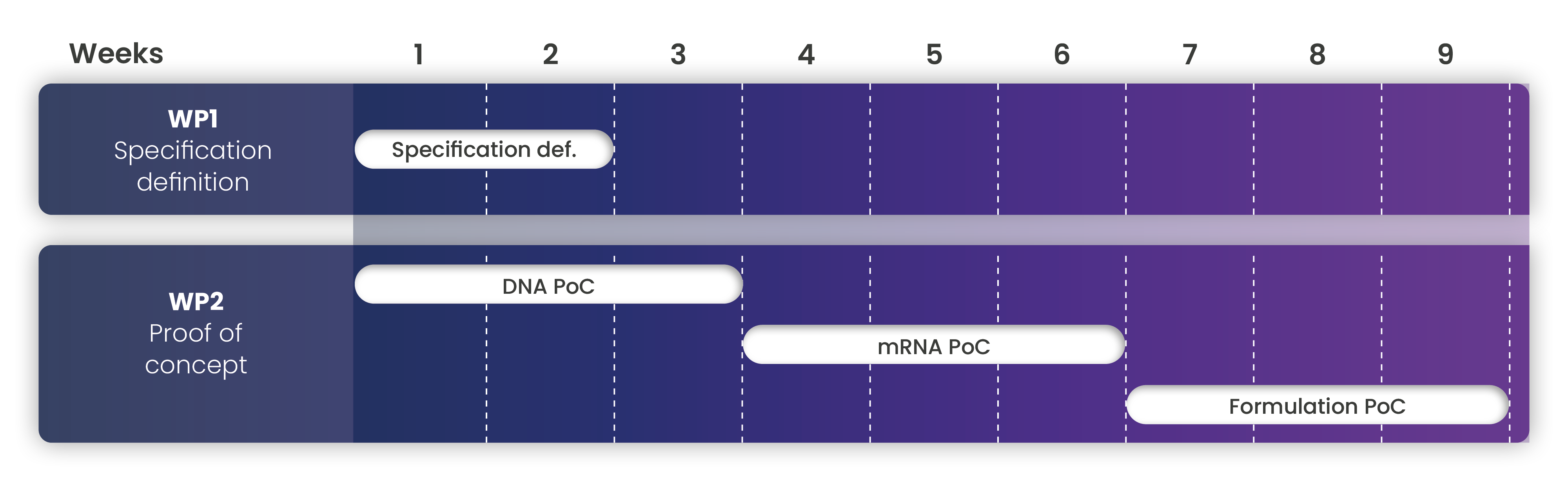
Your proof of concept in only 9 weeks, including analytical testing
Exothera follows Quality by Design approach, where process development is strictly associated with a predefined clinical performance and product quality.
Through the definition of Design Space and the help of statistical methodologies, we can provide a development plan optimized to obtain the best solutions in terms of time and costs, within the quality level our client required.
Your proof of concept in only 9 weeks, including analytical testing
Exothera follows Quality by Design approach, where process development is strictly associated with a predefined clinical performance and product quality.
Through the definition of Design Space and the help of statistical methodologies, we can provide a development plan optimized to obtain the best solutions in terms of time and costs, within the quality level our client required.
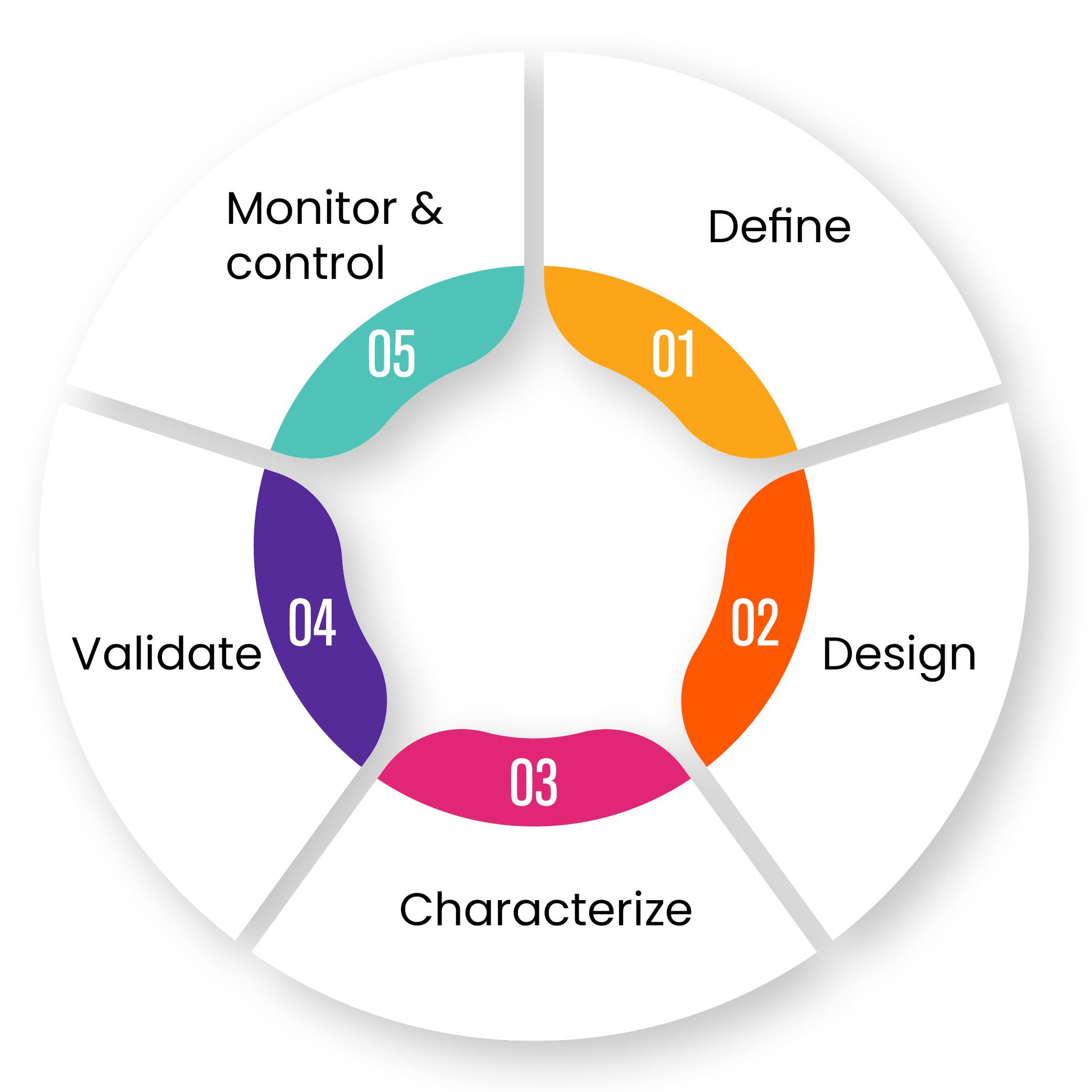
The Quality by Design Methodology
Exothera follows Quality by Design approach, where process development is strictly associated with a predefined clinical performance and product quality.
Through the definition of Design Space and the help of statistical methodologies, we can provide a development plan optimized to obtain the best solutions in terms of time and costs, within the quality level our client required.
For any additional information
Please contact us and we will respond quickly!
The Quality by Design Methodology
Exothera follows Quality by Design approach, where process development is strictly associated with a predefined clinical performance and product quality.
Through the definition of Design Space and the help of statistical methodologies, we can provide a development plan optimized to obtain the best solutions in terms of time and costs, within the quality level our client required.
