Optimized Process Development for Viral-Based Therapies
Accelerate your viral vector therapy with our proven process development expertise.
At Exothera, we specialize in the development and optimization of viral vector manufacturing processes, helping you transition seamlessly from proof of concept to clinical scale.
Please note:
While we offer full process development for viral-based therapies (AAV, adenovirus, lentivirus, etc.), our RNA-based manufacturing does not require process development due to the streamlined nature of our Ntensify® platform.

Smart & Agile Process Development Strategy
Exothera’s multidisciplinary team merges upstream and downstream viral vector expertise with a Quality by Design (QbD) approach to create cost-effective, high-performance manufacturing processes tailored to your therapy’s specific needs.
With more than 20 years of experience within the team, we optimize every step of development—from small-scale proof of concept to full clinical trials—using the latest analytical methods and bioproduction technologies.
Our Services Include:
Plasmid Design & Production (via preferred 3rd partner)
Upstream (USP) & Downstream (DSP) Process Optimization & Development
Analytical Transfer, Bridging & Development
Cell Line & Media Optimization / Re-selection
Manufacturing of Non-GMP & Toxicology Materials
Process Scale-up & Optimization
Formulation Development & Feasibility Studies
Dispensing into Vials, Storage & Product Delivery
Viral Vector Process Development Workflow
Upstream Process (USP) Development
We develop and optimize both adherent and suspension cell culture processes, ensuring high-yield and scalable viral vector production.
Through affordable and rapid proof of concepts, we can perform feasibility studies in shake flasks (suspension) or cell factories (adherent) of your latest discovery.
We perform suspension screening studies with DASGIP® Parallel Bioreactor Systems by Eppendorf, to optimize the number of experiments and boost the statistical value of the data.
We can adapt your existing process and design a cost-effective scale-up thanks to our experience with scale-X™ platform by Univercells Technologies.
Key Technologies:

Scale-X™ Platform (Univercells Technologies)
(for seamless scale-up from 2.4m² to 30m²)

DASGIP® Parallel Bioreactor Systems
(for suspension screening & optimization)

Biostat® RM (Wave Bioreactor)
(for early-stage development from 10L)
Quality by Design (QbD) & Design of Experiment (DoE)
We implement a Quality by Design (QbD) approach, ensuring that process development aligns with predefined clinical performance and product quality standards.
Our Design of Experiment (DoE) Methodology
STEP 1
Define key parameters & experimental strategy
STEP 2
Conduct optimized experiments & collect data
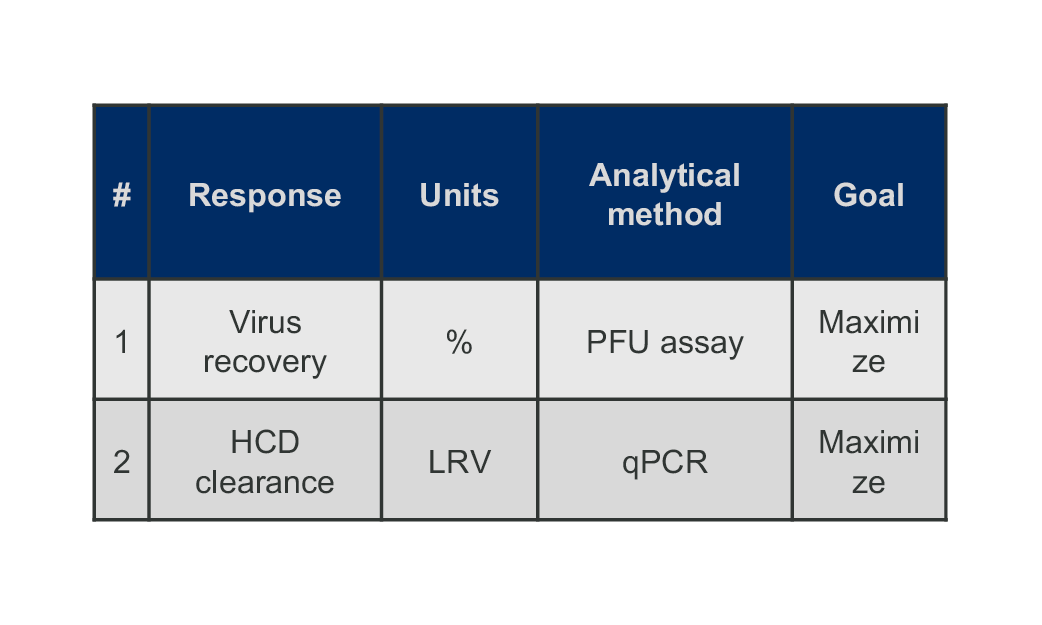
STEP 3
Build predictive models for process optimization
By applying statistical methodologies, we reduce the number of experiments needed while maximizing data insights—delivering a robust and efficient development pathway for your viral vector therapy.
Downstream Process (DSP) Development
We provide advanced purification strategies tailored to your therapy, leveraging best-in-class clarification, filtration, chromatography, and formulation technologies.
Our deep knowledge of the market and the latest technologies allows us to select the best devices and consumables for the success of your process development.
Key Technologies:

PendoTECH Filter Screening System™
(for seamless scale-up from 2.4m² to 30m²)

Repligen KrosFl
(for concentration & formulation development)

ÄKTA avant (Cytiva)
(for chromatography screening & process refinement)

Contichrom CUBE 100 (ChromaCon)
(for continuous chromatography & process intensification)
Why Choose Exothera?
-
Viral Vector Expertise: Backed by team members with 20+ years of hands-on expertise in AAV, adenovirus, lentivirus, and other viral vectors
-
End-to-End Support: From early-stage feasibility studies to full-scale clinical manufacturing.
-
Innovative Technologies: Access to industry-leading USP & DSP platforms.
-
Regulatory-Ready: Development strategies aligned with global GMP & regulatory standards.
-
Cost-Effective Solutions: Optimized scale-up strategies to reduce costs & accelerate timelines.
Looking for more information?
Let’s discuss how Exothera’s viral vector process development capabilities can drive your therapy’s success.