MARKET REPORT
Timeline of viral vector-based Cell & Gene therapy approvals
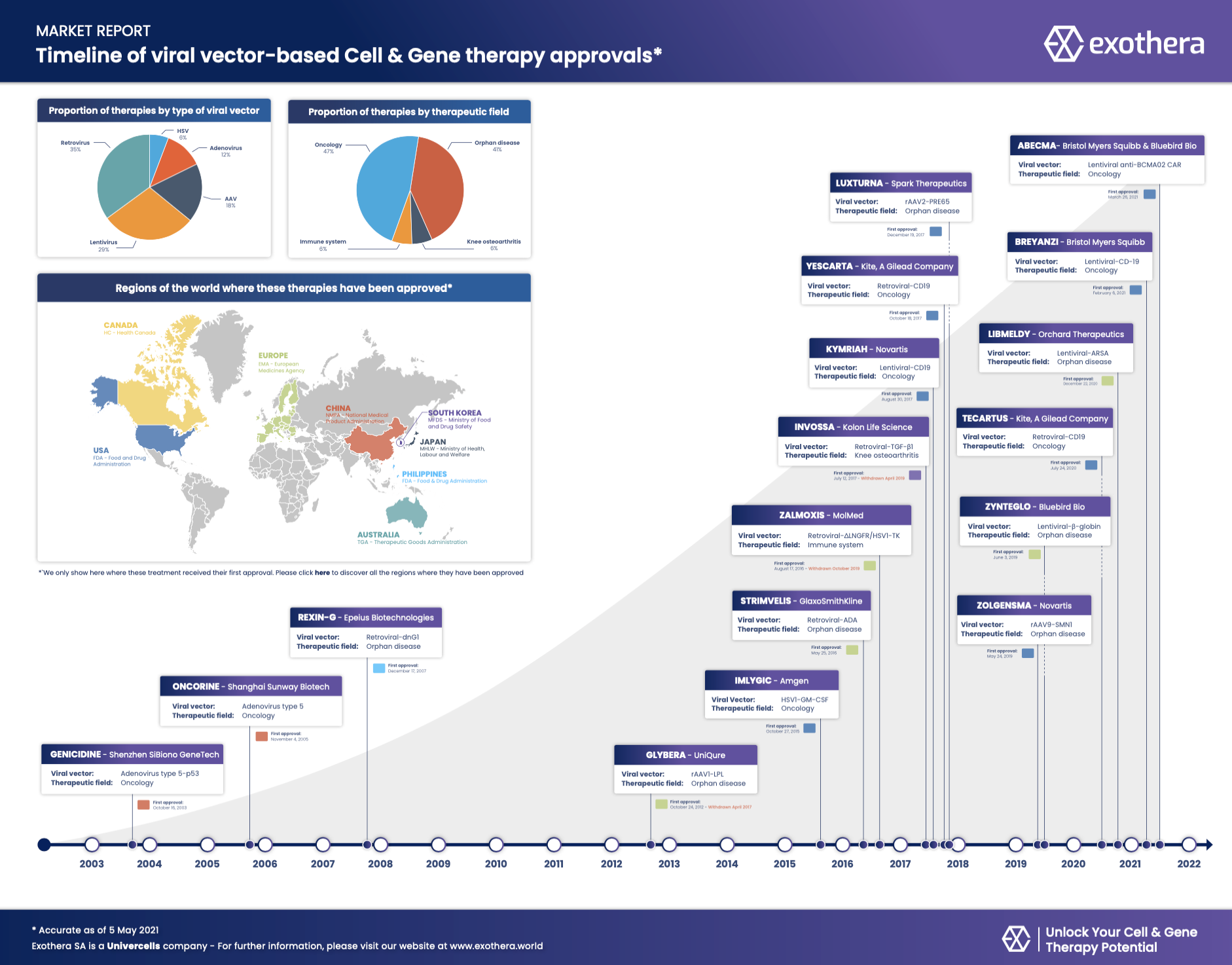
With the approval of the ABECMA therapy last March, there are now 17 cell and gene therapy products*. Although this is very low compared to the number of clinical trials launched during this 18-year period, one can easily see in this infographic that this number has ramped up considerably in the last few years.
But taking a viral vector-based therapy from pre-clinical development to clinical first-in-human studies is still a challenging task. Exothera’s mission is to bring new products to market with efficient and robust processes to make these products rapidly available to as many patients as possible.
* Sources: Alliance for Regenerative Medicine and originators’ websites