By Hanna P. Lesch, Chief Technology Officer, Emilie Gateau, Platform Development Manager, and Michele Marini, Scientific Marketing & Communication associate.
The CGT Market
Gene and cell therapy market is growing rapidly worldwide, and the viral vector manufacturing industry is crucial for the success of these revolutionary treatments. Viral vectors are efficient gene and cell therapy carriers due to their ability to easily enter human cells and because of their recombination flexibility. Projections suggest viral vector manufacturing will grow at a rate of 20% year-on-year through 2025 (CRB Horizons: Cell and Gene Therapy Report, 2020). However, to achieve widespread uptake of gene and cell therapy, the viral vectors industry needs to solve several challenges to obtain cost-efficient manufacturing processes, like scalability of transfection and infection processes. The standard approach to produce a viral vectorbased gene or cell therapy starts with transient expression using transfection or infection of native cell lines. Viral genes (e.g., genes for virion particle’s translation and assembling) and the therapeutic transgene are inserted into the cell’s nucleus through co-transfection or co-infection (Figure 1).
The process becomes even more complex when a helper plasmid or a helper virus is required, as with adeno-associated viruses (AAVs). The transient expression strategy is rapid and flexible during development, but it leads to batch-to-batch variability and high manufacturing costs at scale, due to the significant quantity of extremely pure plasmids (or viral seeds) required. Moreover, the cost of plasmids and viral seeds is likely to increase in the future when regulatory entities make GMPgrade raw materials mandatory for clinical trials and production. Packaging cell line Producers are seeking solutions where transient transfection is limited or completely avoided. Packaging cell lines are cells that are stably transfected to express the proteins for virion particle production and assembly. Only the therapeutic transgene and helper gene information needs to be provided through transfection or infection (Figure 1).
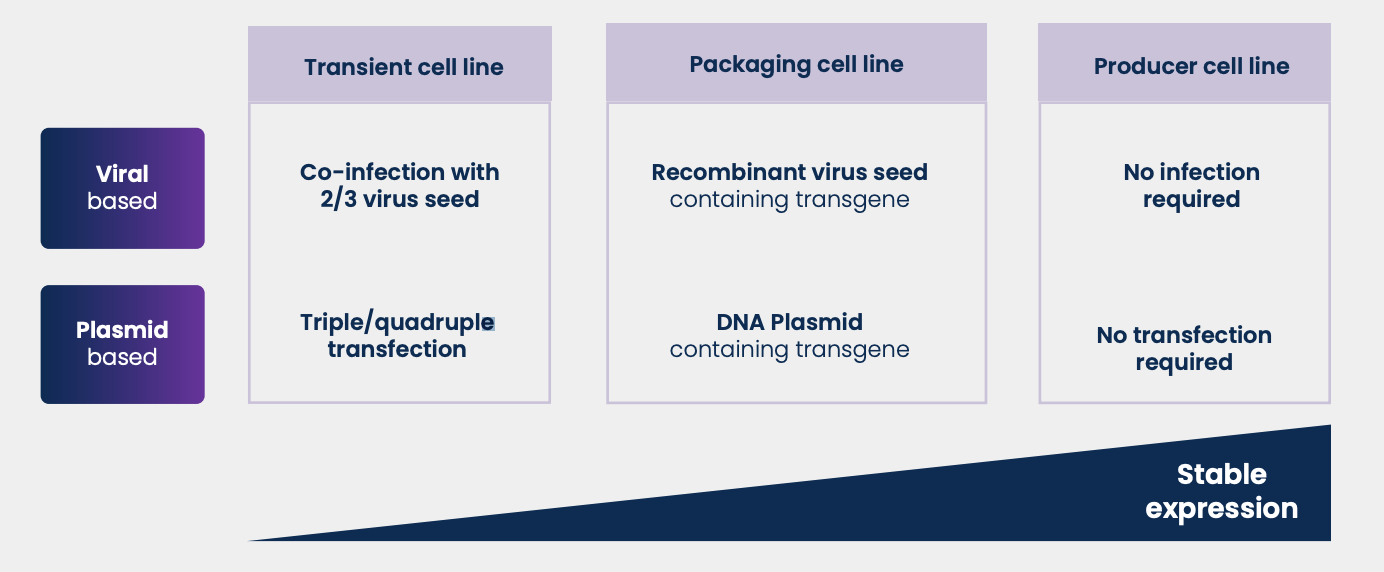
Figure 1 – Cell line strategies for gene and cell therapy manufacturing.
Viral gene expression can be constitutive or induced by an external stimulus. The inducer (a chemical, like doxycycline (Dox) or physical stimulus, like temperature) interacts with the gene promoter to trigger or amplify the expression of viral genes inserted into the cell genome. The transcription boost ensures a high viral vector yield. Compared to the use of native cell lines, packaging cell lines reduce the number of plasmids and viral seeds required and lower batch-to-batch variance. In addition, the same packaging cell lines can be used to produce different gene and cell therapies and are already available as commercial cell lines. However, therapeutic and helper genetic information still needs to be provided and, in the case of inducible packing cell lines, the cost of the inducer and its removal during the purification stage can be significant.
Stable Cell Line
To overcome the scalability limitations of packing cell lines, many producers are developing stable producer cell lines. Producer cell lines are cell lines that are stably transfected with all genetic elements needed for the virion production including the therapeutic transgene and helper genes.
No transient transfection is required (Figure 1) resulting in a major impact on production costs and increased batch-to-batch reproducibility. The downside of producer cell lines lies in the development step. Each gene or cell therapy will require a dedicated cell line development campaign which can last up to 16 months. This explains the low number of commercial producer cell lines available in the market today.
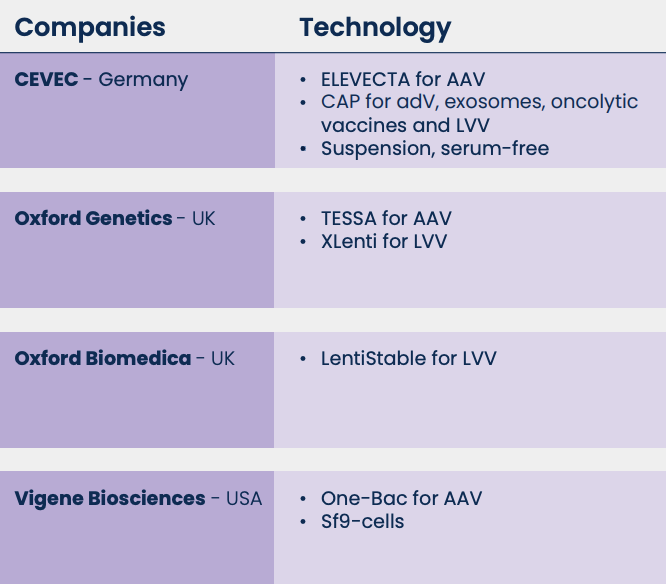
Considering the massive investments in viral vector-based gene and cell therapy, we can envisage new high-throughput techniques that will allow faster and cheaper development of packaging and producer cell lines. For instance, site-specific recombination systems, like Gateway technology (from Thermo Fisher Scientific) for the integration in pre-characterized chromosomal loci of AAVs, can reduce the time of the clone selection step and reduce the development time. Moreover, companies are also providing platforms for agile stable cell line generations (Table 1).
Transient transfection of native cell lines provides fast track to clinic and thus still remains the most used approach where a strategic decision is prioritizes fast product safety and efficacy testing over manufacturing optimization. Longerterm manufacturing decisions should focus on reduced cost of goods with stable cell lines. Until that, strategies to reduce the cost of transient transfection are required, such as smart critical process parameter optimization performed through a Design of Experiments (DoE) approach supported by thorough analytics and deep process understanding.