By Hanna P. Lesch, Chief Technology Officer, Emilie Gateau, Platform Development Manager, and Michele Marini, Scientific Marketing & Communication associate.
The success of viral vector-based gene and cell therapy passes by rapid and smart scale-up technology, from development to production scale. Modern scalable bioreactors represent strong candidates thanks to high degree of automation, monitoring features and low contamination risk.
Gene and cell therapies are innovative biological therapeutics developed especially for cancer and inherited monogenetic diseases. They represent an alternative to chronic therapies making a “one-shot” cure for many diseases and conditions a reality. However, process development time and manufacturing costs issues are slowing down the spread of gene and cell therapies.
The standard strategy is to encapsulate the therapeutic gene in a carrier, usually a viral vector, then produce this in cell culture. However, the viral vector production industry still partially uses open and manual processes, while gene and cell therapy require closed, automated, and scalable platforms, which should speed up development and reduce manufacturing costs.
Modern scalable bioreactors allow rapid scaleup from development to production scale. They ensure a high degree of automation and monitoring which reduces costs, manpower, and contamination risks. They can be easily paired with continuous processing steps – like tangential flow filtration – to increase cell density and viability. Many solutions are available on the market for both adherent and suspension cell culture and in disposable formats.
Cell culture method: adherent vs suspension
The selection of the bioreactor depends on the cell culture method: adherent, where cells need a solid substrate to grow as a monolayer, or suspension, where the cells can grow free-floating in the medium. Most mammalian and insect cell lines are naturally anchorage-dependent with only a few exceptions, like hematopoietic cells. The yield, footprint and scalability will therefore be limited by the available growth surface. The use of flatware flasks allows easy cell monitoring under an inverted microscope, but periodic subculture steps are required. In addition, dissociation from the substrate, for manipulation or harvesting, requires a mechanical or enzymatic action.
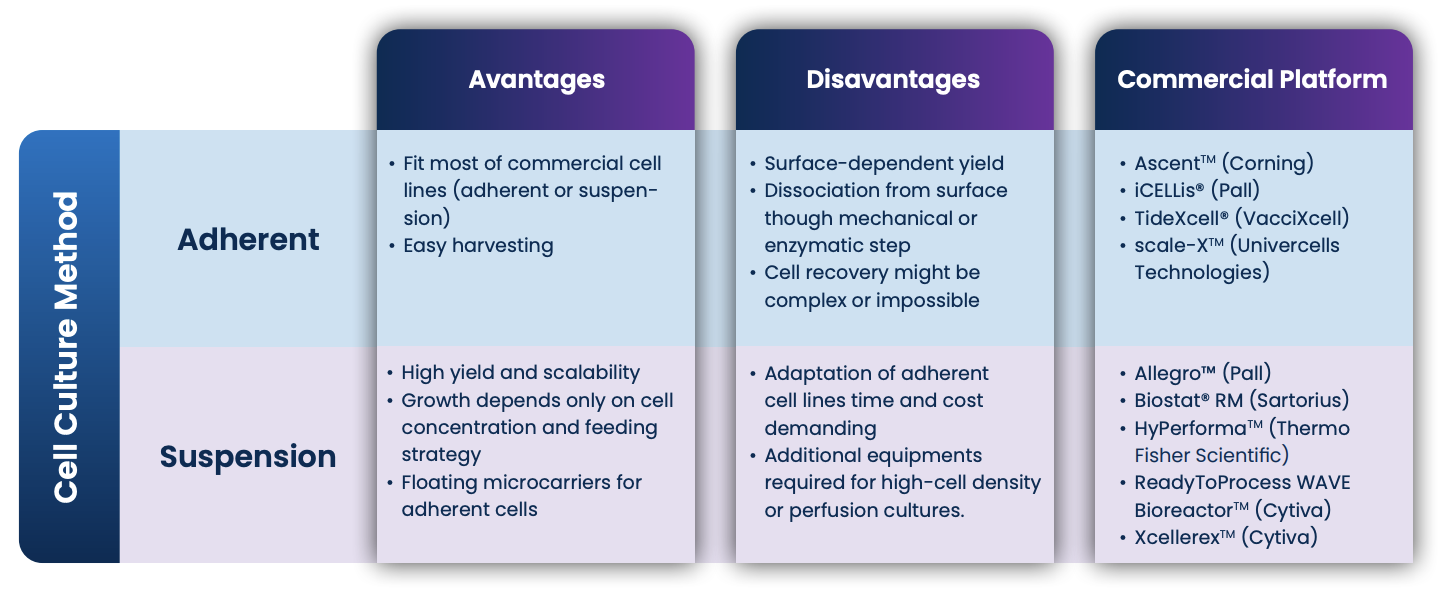
Table 1 – Summary of cell culture method characteristics and most commonly used commercial platform
The use of microcarriers as a substrate avoids having to adapt adherent cells for suspension but has some disadvantages. Microcarriers are difficult to handle, and their removal requires a dedicated purification step. Moreover, high shear stress and turbulence generated in stirred-tank bioreactors (see next section) might jeopardize adherent cells’ capacity to grow and survive.
Suspension cells float in the medium where growth is limited only by cell concentration and the feeding strategy. Subculture is easier and cell monitoring is possible through daily sampling or using a cell monitoring probe, available for most bioreactor setups. Even if no dissociation step is needed, some cell lines could present a tendency to aggregate requiring the use of cell strainers or the addition of anti-clumping reagents which are often incompatible with the transfection reagent. Most adherent cell lines can be adapted to grow in suspension, but adaptation can be an expensive and time-consuming process. The market provides some ready-to-use adapted suspension cells, that often come with royalties and/or license fees.

Commercial bioreactors
Highly scalable bioreactors – such as iCELLis® (Pall), TideXcell® (VacciXcell), AscentTM (Corning) or scale-XTM (Univercells Technologies) – for adherent cell culture are available on the market. These devices have proven to be efficient for viral vector manufacturing and offer a singleuse approach, continuous monitoring, excellent cell growth, smooth perfusion to support high cell densities, rapid media exchange and easy harvesting. Adherent fixed-bed bioreactors have also been adapted for suspension cells culture.
Wave-type suspension bioreactors – like the ReadyToProcess WAVE 25 Rocker (Cytiva) or the Biostat® RM (Sartorius) – are other options that are available, but scalability is limited. A singleuse bag is placed on a rocking motion device and filled with cell culture fluid. Smaller wave type bioreactors contain integrated floating filterbased perfusion capability. The waves provide the mixing of the fluid and oxygen transfer. With scale available between < 1L to 200 L, the rockingmotion bioreactor are predominantly used for seed train steps.
Stirred-tank bioreactors – like Allegro™ (Pall), XcellerexTM (Cytiva) Hyperforma (Thermo Fisher scientific) or Biostat STR® (Sartorius) – are largely used for suspension, with scalability up to 2000 L. Cells float in media which are constantly agitated to guarantee homogenous distribution of all the nutrients. To support high cell densities, the perfusion will require an additional device like Alternative Flow FIltration (ATF) which can bring additional shear stress to the cells or viruses and present a risk of clogging at high cell density.
Conclusion
Gene and cell therapies based on viral vectors are promising life changing treatments, but their success requires a change in paradigm from the traditional biopharma industry approach, starting with cell culture strategy and scalability. Modern scalable bioreactors can fast track the design of new, closed, secure, and automated platforms for the development and production of gene and cell therapy both in adherent and suspension cell culture. Adherent-based bioreactors can host most of the cell lines on the market, with easy perfusion and harvesting, but scalability is surface-dependent. Suspension bioreactors allow high yield and better scalability, but they present more challenges when implementing perfusion and not all commercially available cell types can be adapted to suspension conditions.


