Ready-to-go nucleic acid production process
The platform incorporates DNA and RNA manufacturing together with formulation, along with critical reagent supply to address the numerous challenges involved when developing mRNA-based vaccines or therapeutics, from sequence design up to commercial production (e.g., 100 million doses of vaccines)

Ready-to-go nucleic acid production process
The platform incorporates DNA and RNA manufacturing together with formulation, along with critical reagent supply to address the numerous challenges involved when developing mRNA-based vaccines or therapeutics, from sequence design up to commercial production (e.g., 100 million doses of vaccines)

Capitalize on exoREADY process
Nucleic acid
dedicated platform
- EFirst continuous-batch RNA manufacturing platform in the world
- EPartnered with the inventors of the technology
- EDedicated to manufacturing of DNA, RNA and LNPs - Can be used at every step of your journey: from discovery up to commercial GMP manufacturing
Ntensify™ equipment
- ENew industry gold standard in RNA manufacturing
- EEngineering principles of intensification and chaining for highest quality product
- ESingle small footprint system that integrates all process steps accelerating product development and significantly reducing CoGs
- EHigh quality at high speed, consistent and multiple- purpose production equipment
Ready to use
technology
- EPre-developed analytical toolkit to minimize analytical development
- EOff-the-shelf reagent premixes for improved production and higher yields
- EIn vivo validated with multiple RNA constructs to de-risk your product development
Go faster through the clinical phase at higher quality
We go from antigen definition to 1st R&D batch production in less than five months
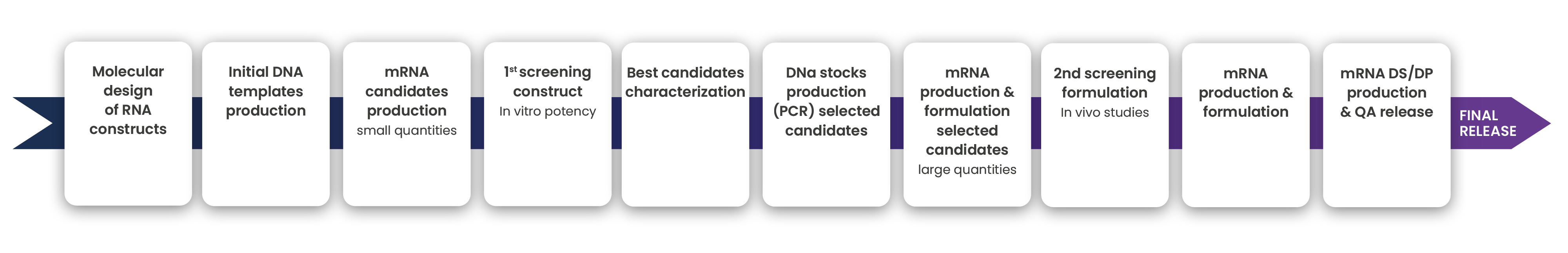
Scaling manufacturing to GMP
We selected the Nftensify™ technologies to take nucleic acid-based processes from small scale R&D to large scale clinical and commercial manufacturing seamlessly. By using this groundbreaking technology, pre-clinical or clinical development is already at the final scale, which accelerates process and product development and significantly speeds up deliver to patients.