A smart and agile development strategy
Step by step solution: from proof of concept to clinical scale
Our development approach
Exothera’s multidisciplinary structure merges upstream and downstream viral vectors expertise with a Quality by Design (QbD) approach to meet your needs.
From small scale to clinical trials we provide comprehensive quick and cost-effective development solutions, supported by the latest analytical methods

Development process flow

Technologies and expertise
Our team of experts counts more than 20 years of upstream and downstream development experience. Exothera can work with you to develop the best process for your product, with several solutions for adherent and suspension processes and with the best-in-class purification techniques and systems.
Upstream Development
Through affordable and rapid proof of concepts, we can perform feasibility studies in shake flasks (suspension) or cell factories (adherent) of your latest discovery.
We perform suspension screening studies with DASGIP® Parallel Bioreactor Systems by Eppendorf, and to optimize the number of experiments and boost the statistical value of the data.
We can adapt your existing process and design cost-effective scale-up thanks to our experience with scale-X™ platform by Univercells Technologies.




Downstream Development
Our team can meet your requests for an efficient development of a purification strategy, thanks to a deep experience with clarification, concentration, chromatography and formulation. We provide best-in-class systems to perform feasibility studies and screening.
We can perform filtration screening with PendoTECH Filter Screening System™ by PendoTECH and chromatography screening with ÄKTA avant by Cytiva to optimize your purification process. We can adapt your traditional chromatography steps to continous chromatography thanks to our experience with Contichrom CUBE by ChromaCon. Our deep knowledge of the market and the latest technologies allows us to select the best devices and consumables for the success of your process development.

PendoTECH Filter Screening System™


ÄKTA avant

Contichrom CUBE 100
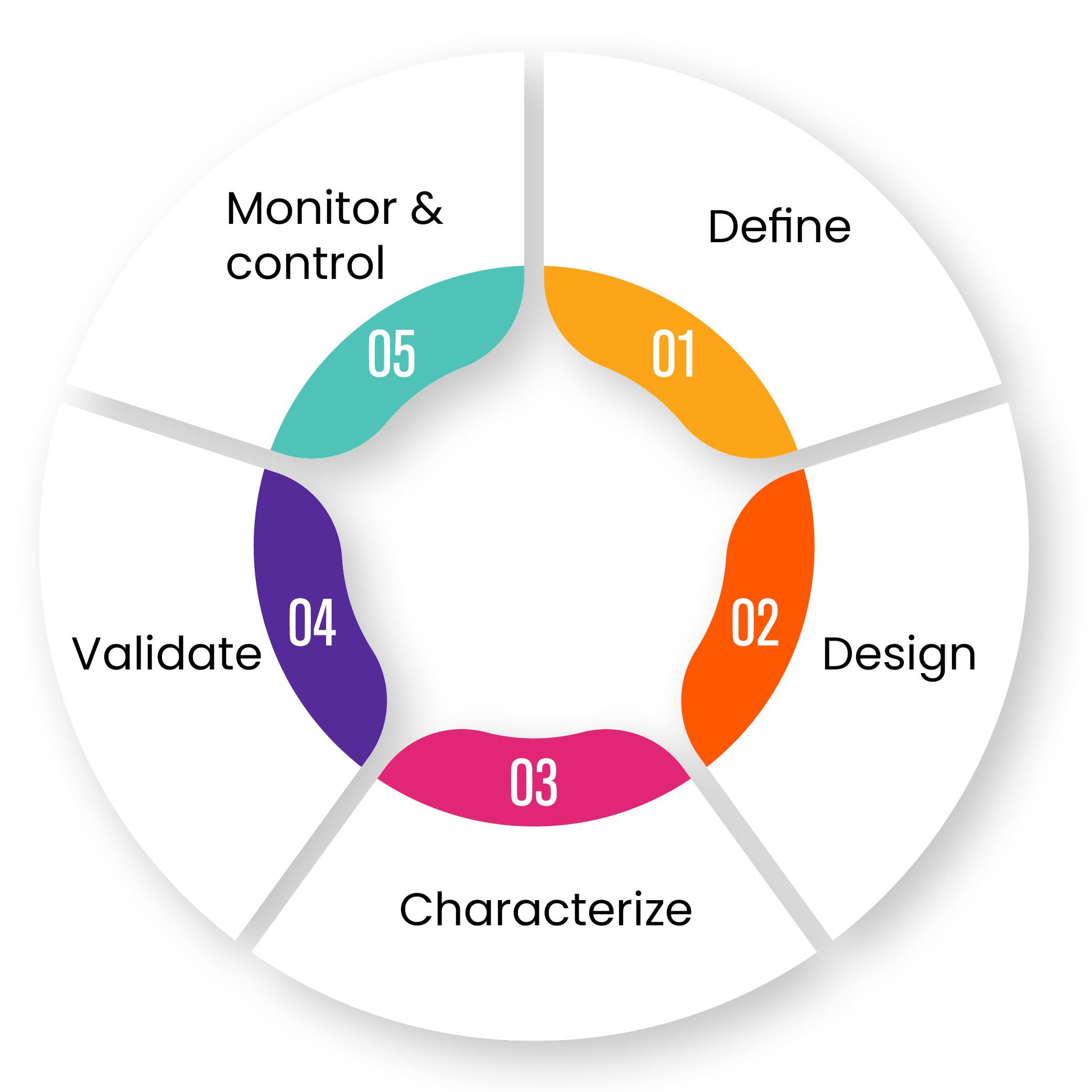
The Quality by Design Methodology
Exothera follows Quality by Design approach, where process development is strictly associated with a predefined clinical performance and product quality.
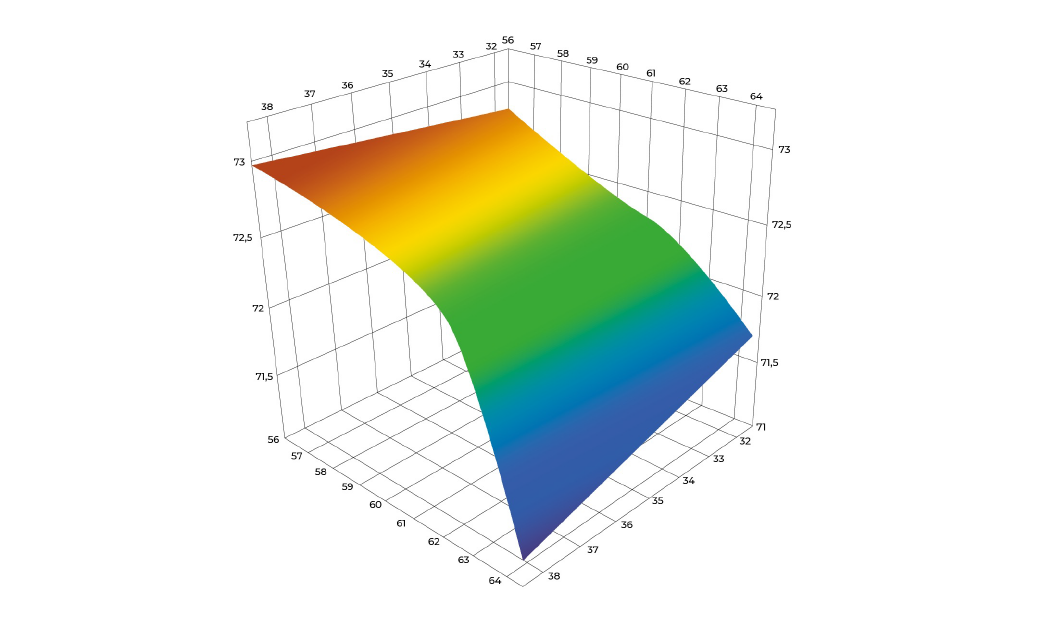
Through the definition of Design Space and the help of statistical methodologies, we can provide a development plan optimized to obtain the best solutions in terms of time and costs, within the quality level our client required.
Design of Experiment
At Exothera, we use Design of Experiment (DoE) to manipulate multiple factors and parameters at the same and identify possible interaction between them, otherwise undetectable with a traditional approach, with an impact on the number of experiments to perform. Our data scientists will support development team to define a set of experiment that can provide all the information for a successful characterization and optimization of the final process.
STEP 1
Define the DoE objectives and select the parameters of the study, which lead to select the DoE methodology
STEP 2
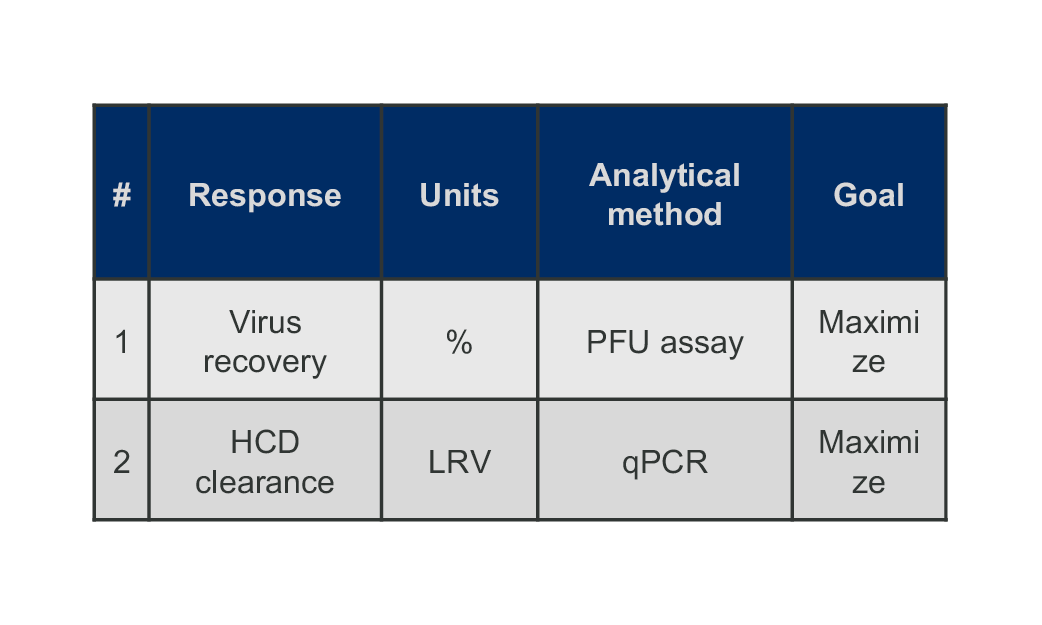
Perform the experiment and collect the factor response
STEP 3
Build the predictive model within the design space