Fast-track your therapy or vaccine to patients, without compromising quality
- Harness game-changing capabilities powered by the world-first continuous-batch RNA production platform, Ntensify™ – developed in-house by our sister company Quantoom Biosciences
- Utilize our optimized platform to produce small-batch quantities for early clinical work to large-scale vaccine or therapeutic production
- Progress your project from DNA to RNA at double the speed of traditional manufacturing processes with Ntensify™
- Harness standardization and automation to streamline workflows, enhance reproducibility, and reduce development timelines by up to half
- From discovery to GMP, our ecosystem of solutions is designed to support you at every stage, enabling you to eliminate common process development and scale-up activities for a smooth journey from concept to market
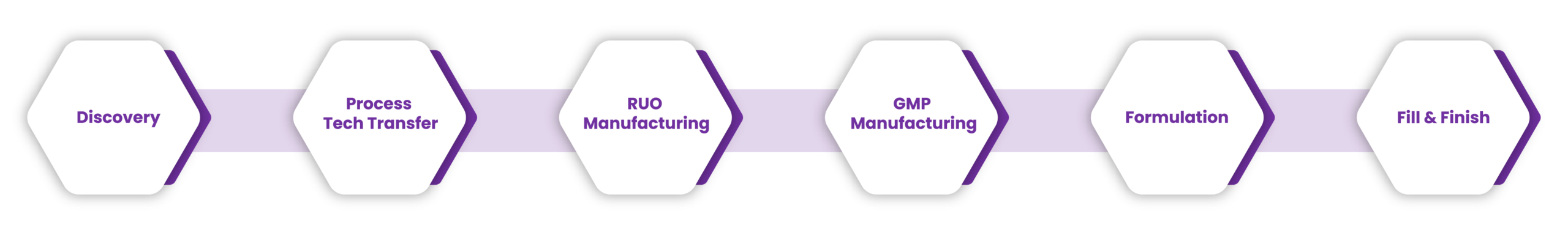
Discovery
Starting from a known antigen, we can provide support for the design and production of mRNA-based therapeutics.
Exothera’s discovery services for RNA therapy developers involved in early-stage discovery (antigen definition) provides access to RNA expertise, process knowhow and production systems at each stage of the discovery workflow, from RNA design, and manufacture up to in vitro screening for potency and manufacturability demonstration, all from a single provider.
- EAntigen definition
- EConstruct design
- EConstruct screening
- EFormulation screening
Process Tech Transfer
We can convert your existing RNA production method to our standardized and optimized manufacturing process at any stage of your project.
Harnessing our exoREADY process, we can help you achieve increased quality, performance, speed, and lower CoGs – all aligned with your specifications and expectations.
- EProduction method review and data analysis
- ERisk and gap identification
- ETechnology comparison
- ECost modelling
RUO Manufacturing
We offer fast, high-quality and cost-effective RNA production at ug, mg, or g scales for your research or preclinical needs.
Available across the US and EU, we’re ready to help set up a seamless transition to produce larger material quantities for phase I and beyond.
- EmRNA production & formulation of selected candidates
- EConstruct specific analytical method development
- EAnalytical characterization of purified and formulated candidates thanks to our extensive analytical assays offer.
GMP Manufacturing
We support your GMP production at any stage including DNA, RNA, LNP and Fill Finish from clinical through to commercial scales. With robust quality and regulatory expertise, we help to ensure the smooth advancement of your product through the clinic.
- EManufacturing of RNA product: from ug quantities to 5g/day of purified RNA
- ECMC support
- EGMP manufacturing, testing and release services
- EFormulation and encapsulation in LNP
- EFill & Finish
- EStorage
- EStability studies on Drug Substance and Drug Product
Analytics
Our analytical capabilities cover the whole spectrum from DNA, RNA and LNPs. We support analytical development from discovery to bridging capabilities for preclinical and clinical processes, up to quality control during GMP manufacturing.
- EDevelopment of new analytical methods
- ERoutine analysis of R&D samples based on our standard analytical qualified methods catalog
- EAnalysis of GMP samples based on our QC’s validated method catalog

Discovery
Starting from a known antigen, we can provide support for the design and production of mRNA-based therapeutics.
Exothera’s discovery services for RNA therapy developers involved in early-stage discovery (antigen definition) provides access to RNA expertise, process knowhow and production systems at each stage of the discovery workflow, from RNA design, and manufacture up to in vitro screening for potency and manufacturability demonstration, all from a single provider.
- EAntigen definition
- EConstruct design
- EConstruct screening
- EFormulation screening
Process Tech Transfer
We can convert your existing RNA production method to our standardized and optimized manufacturing process at any stage of your project. Harnessing our exoREADY process, we can help you achieve increased quality, performance, speed, and lower CoGs – all aligned with your specifications and expectations.
- EProduction method review and data analysis
- ERisk and gap identification
- ETechnology comparison
- ECost modelling
RUO Manufacturing
We offer fast, high-quality and cost-effective RNA production at ug, mg, or g scales for your research or preclinical needs. Available across the US and EU, we’re ready to help set up a seamless transition to produce larger material quantities for phase I and beyond.
- EmRNA production & formulation of selected candidates
- EConstruct specific analytical method development
- EAnalytical characterization of purified and formulated candidates thanks to our extensive analytical assays offer.
GMP Manufacturing
We support your GMP production at any stage including DNA, RNA, LNP and Fill Finish from clinical through to commercial scales. With robust quality and regulatory expertise, we help to ensure the smooth advancement of your product through the clinic.
- EManufacturing of RNA product: from ug quantities to 5g/day of purified RNA
- ECMC support
- EGMP manufacturing, testing and release services
- EFormulation and encapsulation in LNP
- EFill & Finish
- EStorage
- EStability studies on Drug Substance and Drug Product
Analytics
Our analytical capabilities cover the whole spectrum from DNA, RNA and LNPs. We support analytical development from discovery to bridging capabilities for preclinical and clinical processes, up to quality control during GMP manufacturing.
- EDevelopment of new analytical methods
- ERoutine analysis of R&D samples based on our standard analytical qualified methods catalog
- EAnalysis of GMP samples based on our QC’s validated method catalog