ARTICLE
Serum-based media concerns in viral vector manufacturing
Nowadays the viral vector manufacturing industry is largely dependent on adherent cells and serumcontaining media. Serum is a biological product that contains various constituents essential for cell growth (e.g., hormones, growth factors, additional amino acids, vitamins, trace elements, fatty acids, lipids, etc.). It significantly increases productivity and acts as a buffer for viral particles. It increases the viscosity of the medium and reduces mechanical damages to cells due to shear stress. Serum constituents can also improve viral particle stability, thereby improving titer at harvest. Typical serum concentration in cell culture media varies from 1% to 15%.
Regardless of the benefits, serum raises several issues. Quality assurance concerns, price fluctuations, and ethical dilemmas are pushing the industry towards serum-free media. Although more ethical and consistent, it is still not sufficiently well accepted to take over the market.
In this article, we will discuss the pros and cons of serum-containing and serum-free media, and how to overcome the challenges associated with serum use in viral vector manufacturing.
Fetal Bovine Serum origin and grade
Fetal bovine serum (FBS) (or fetal calf serum, FCS) is the most commonly used serum supplement in cell culture. It is obtained from the blood of unborn calves (fetuses) directly in abattoirs during the slaughtering of pregnant cows and obtained through a strictly regulated purification process. Different grades of FBS are available, based on geographical origin, level of purification and the Good Manufacturing Practices followed during production.
The Geographical Risk Assessment (GRA) assigns a level of security to a country, or a region based on the probability that cows are infected with certain diseases, like BSE (Bovine Spongiform Encephalopathy, or mad cow disease). GBR Level 1 (Table 1) indicates a low risk of animal infection in countries without BSE and other severe diseases, hence FBS from these countries is considered more secure. FBS with a good assessment grade is a guarantee of a single source of serum from a level one region. The International Serum Industry Association (ISIA) controls the traceability of all FBS lots produced.
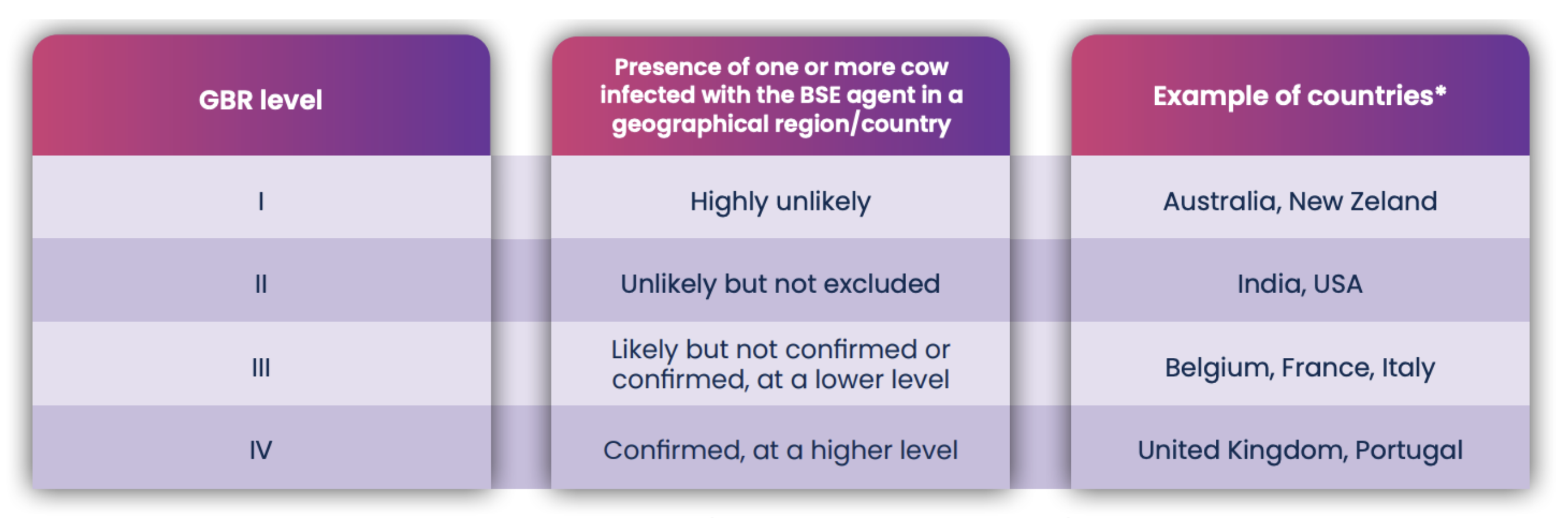
* GBR level based on the latest report from EU commission (click here for more information)
The purification process can impact the FBS grade. Suppliers provide different grades of FBS based on the number of tests and purification steps performed. For example, extra ultracentrifugation may secure an ultra-low IgG content certificate which is relevant when developing immunotherapeutics.
Traditional adherent cell culture is serum dependent
The majority of the original cell lines on the market are traditional anchoring-dependent cells. Each cell line has its own growth characteristics and media requirements. Adherent culture typically requires serumcontaining media since serum contains attachment factors to promote cell anchoring on the substrate. Serum also contains growth supplements, which are missing on most of the widely used basal media, like DMEM (Dulbecco’s Modified Eagle Medium).
From a manufacturing point of view, FBS and other sera raise several concerns. Serum introduces high batch-to-batch variations because it is not chemically defined. It also increases contamination risk and regulatory concerns by introducing animal components to the cell culture. Serum can also complicate the downstream purification of the final product, reducing final yield. Moreover, the price of sera fluctuates every time an unexpected event like a natural disaster, animal epidemic or transportation disruption occurs in the region of origin. Finally, the use of material coming from animals represents an ethical issue that needs to be addressed to stay relevant in the market.
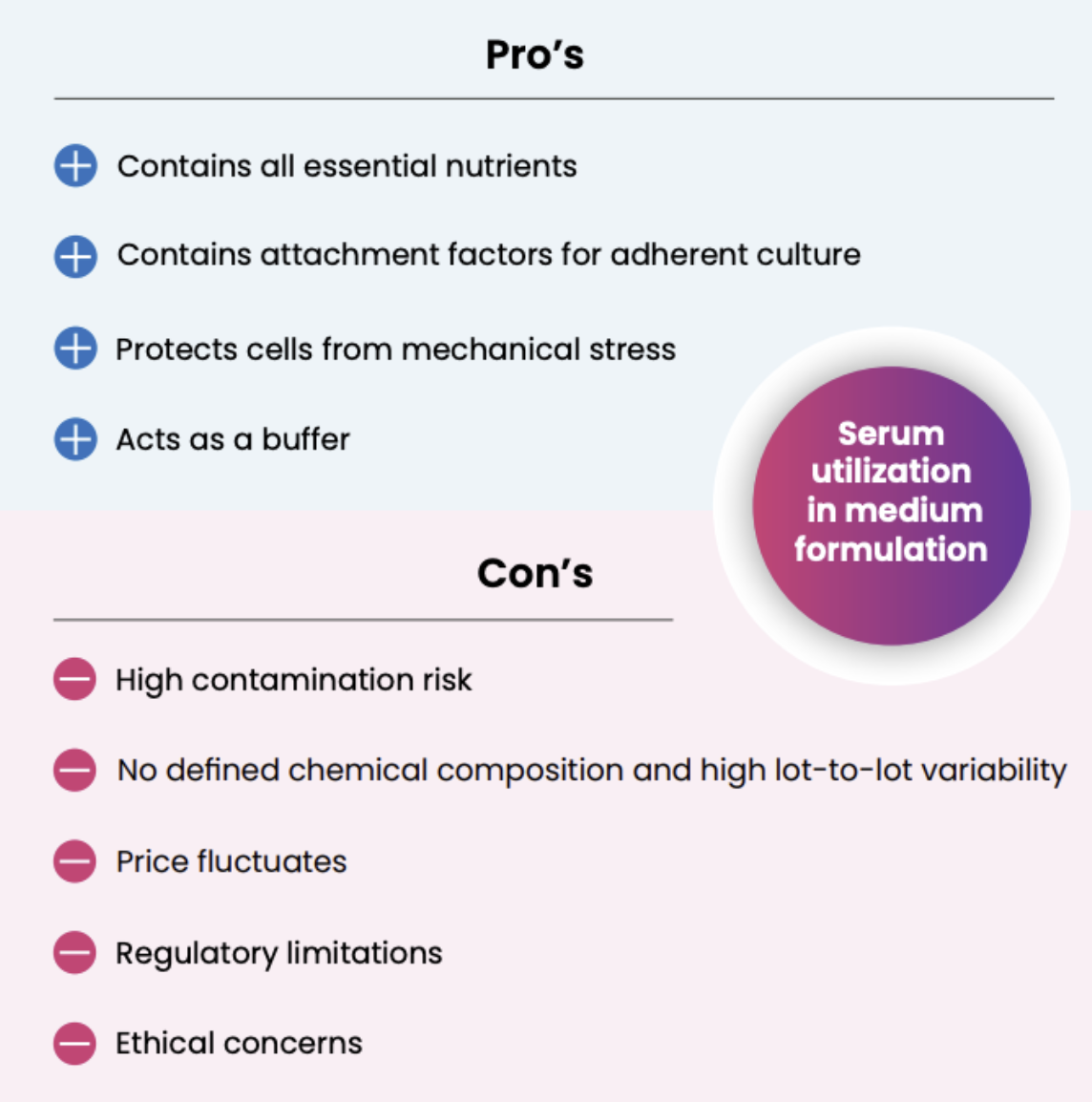
Serum-free media, a way forward for all viral vector production processes?
The industry is pushing towards serum-free media, and many solutions are already available on the market. Serum-free media can contain proteins, but they are labeled as “chemically defined” only if they contain recombinant proteins. Chemically defined media can also be protein-free (no proteins above 10k Dalton) and peptide-free (only low molecular weight constituents below 900 Dalton).
We found a limited number of references to serum-free media being adapted for adherent cell culture. These include BIO-MPM-1 (Sartorius), Pro293TM Serum-free Medium (Lonza), and FreeStyleTM F17 or VP-SFM (Thermo Fisher).
Suspension culture can be performed without serum-containing media because cells float in the media, thus no attachment factors are required. Suspension cell culture might drastically reduce the need for serum in the industry, but few cell lines are naturally non-adherent (e.g., hematopoietic cells) and the adaptation of an adherent cell line to suspension culture is time consuming and costly, as we discussed in our article “Are modern scalable bioreactors the right cell culture strategy for gene and cell therapy success?”. (click here to access the article).
Most of the serum-free media on the market are cell line specific and adapted for suspension cell lines.
For example, HEK293-derived cell lines are widely used in viral vector production and are available commercially. Among the most popular serum-free formulations for HEK293-derived cells are the Ex-CELL 293 (Sigma-Aldrich), FreeStyleTM 293 (ThermoFisher), HyClone SFM4Transfx-293 (Cytiva) and BalanCD HEK293 (Irvine) media. Moreover, many HEK293-derived cells have been adapted for suspension culture.
An increasing number of cell lines adapted to the different commercially available media can be found ready to use. Access to these cell lines often comes with significant license fees or royalties.
Increasing need to perfect medium formulation for specific cell line and product application
Primarily, serum free medium formulations were developed to sustain recombinant protein production processes. Transposed to the production of viral vectors, additional challenges might appear such as incompatibility with transfection reagents or inability to sustain high titer viral production.
The market is responding to this increasing request for formulation adapted for viral vector production and every day new serum-free media, supplements, enhancers, and new cell lines are becoming available, speeding up the media and culture adaptation process.
The media are designed to balance the specific requirement for supplements, like amino acids, surfactants, or insulin-transferrinselenium derivates. Customization of the supplementation strategy may be economically disadvantageous if the costs of additives required to obtain satisfactory yield, particle stability and productivity are too high.
Customization of medium formulation
In case no serum-free media is available for the selected cell line, it is possible to develop a dedicated medium following a pyramid approach as shown in Figure 1. The approach consists of added supplements more and more specific for the cell line selected, to reach a medium that can guarantee the growth and proliferation of the selected cell line.
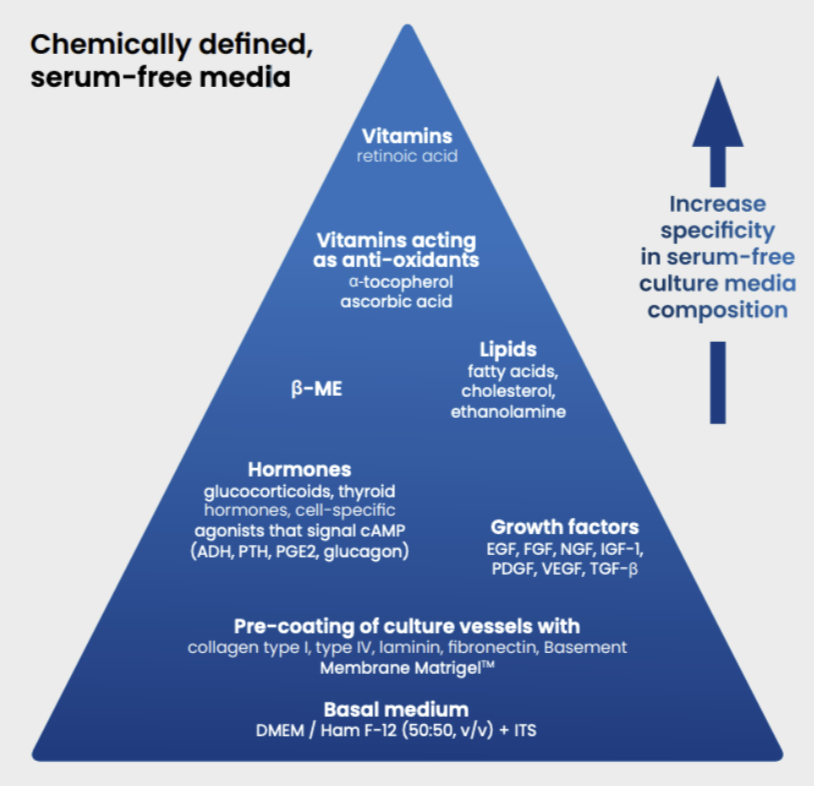
Figure 1 – Media pyramid: a modular approach for the development of serum-free media. Abbreviations: ADH, antidiuretic hormone; EGF, epidermal growth factor; FGF, fibroblast growth factor; IGF-1, insulin-like growth factor 1; ITS, insulin–transferrin– sodium selenite supplement; β-ME, β-mercaptoethanol; NGF, nerve growth factor; PDGF, platelet-derived growth factor; PGE2, prostaglandin E2; PTH, parathyroid hormone; TGF-β, transforming growth factor-b; and VEGF, vascular endothelial growth factor. (Source: DOI: 10.1016/j.tiv.2010.03.016)
A common starting point is a basal media formed by a mixture of Dulbecco’s modified MEM (DMEM), Ham’s Nutrient Mixture F-12 (50:50 v/v), and ITS supplement (insulin, transferrin, and selenium). DMEM is rich in amino acids and F-12 contains trace elements, vitamins, glucose, and other supplements, like zinc sulfate, putrescine, and linoleic acid. Insulin is essential for cell culture and is used extensively in culture media. Transferrin is involved in the transfer of iron into the cells. Selenium is an essential trace element for selenoproteins, which protect cells against oxidative stress.
Further supplements included in the mixture depends on specific cell line properties. These include growth factors, hormones, shear force protectors – serum’s viscosity needs to be replaced, often with a supernatant like poloxamer – protease inhibitors, vitamins, proteins, etc.
Other supplements can be included in the mixture to improve manufacturing quality and quantity. For example, in the case of viral vector manufacturing, serum-free media also contain stabilizers for the viral particle, like lipids.
In all cases, the selection of the most suitable medium formulation and adaption is a timeconsuming and challenging process for which the investment need to be carefully considered.
Conclusion
The viral vector manufacturing industry is still mostly serum dependent, but this paradigm should change for the large-scale production of viral vector-based biotherapeutics like cell and gene therapy. Serum-free media can facilitate scalability, with benefits in terms of safety, batch-to-batch variation, and costs. However, this technology shift requires a series of investments in the development of both serum-free media and suspension cell lines. Although adaptation can be time consuming and costly, in the long run, the shift to serum-free protocols will support a successful, affordable, and ethical scale-up of cell and gene therapy manufacturing processes.
Download the PDF version of the article
he viral vector manufacturing industry is largely dependent on adherent cells and serum-based media. Serum provides constituents essential for cell growth and viral particle stability, but it increases the risk of contamination, lot-to-lot variation and ethical concerns.
Serum-free media can mitigate most of these issues, but development and optimization campaigns are required to overcome the advantages provided by serum.

