In-depth review of your current viral vectors process with exoScan
Our process analysis methodology, called exoSCAN, aims to achieve an in-depth review of your current process and its clinical and commercial potential within 8 weeks. The comprehensive development plan identifies areas for optimization that can fast-track the clinical phase, prepare for competitive commercial launch and act as a financial roadmap for fundraising or board endorsement.

In-depth review of your current viral vectors process with exoScan
Our process analysis methodology, called exoSCAN, aims to achieve an in-depth review of your current process and its clinical and commercial potential within 8 weeks. The comprehensive development plan identifies areas for optimization that can fast-track the clinical phase, prepare for competitive commercial launch and act as a financial roadmap for fundraising or board endorsement.

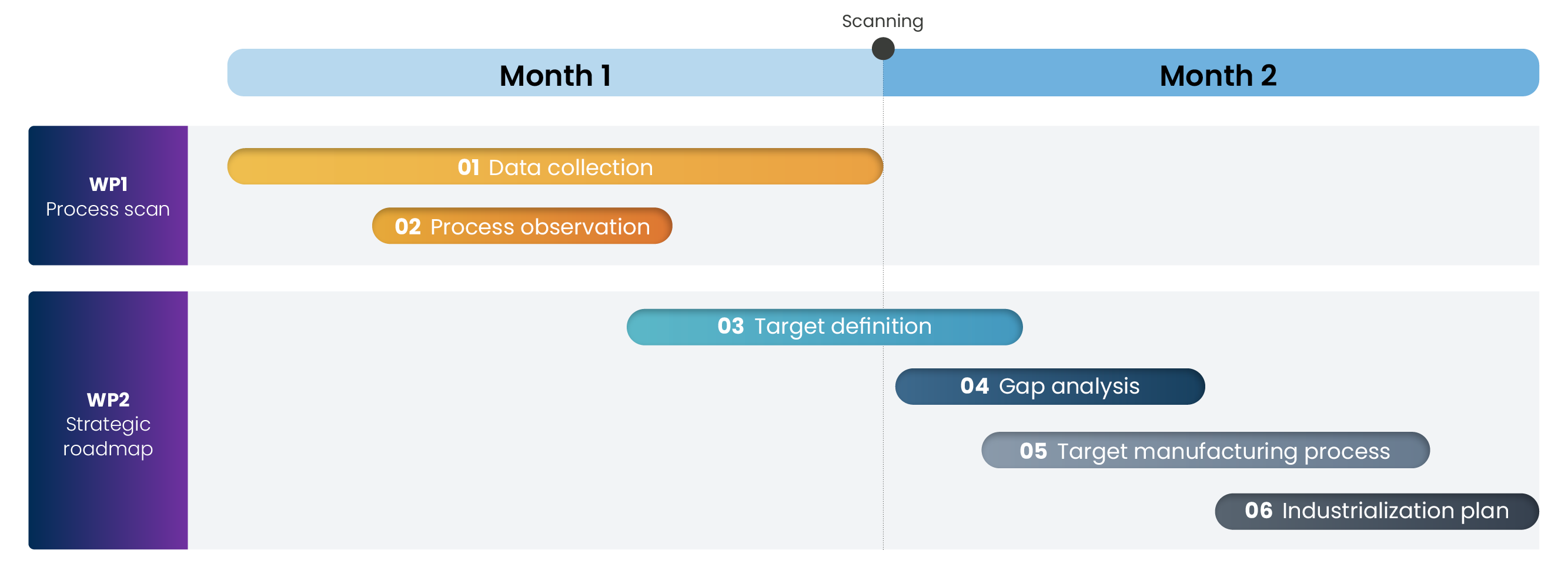
Your strategic roadmap in only 2 Months
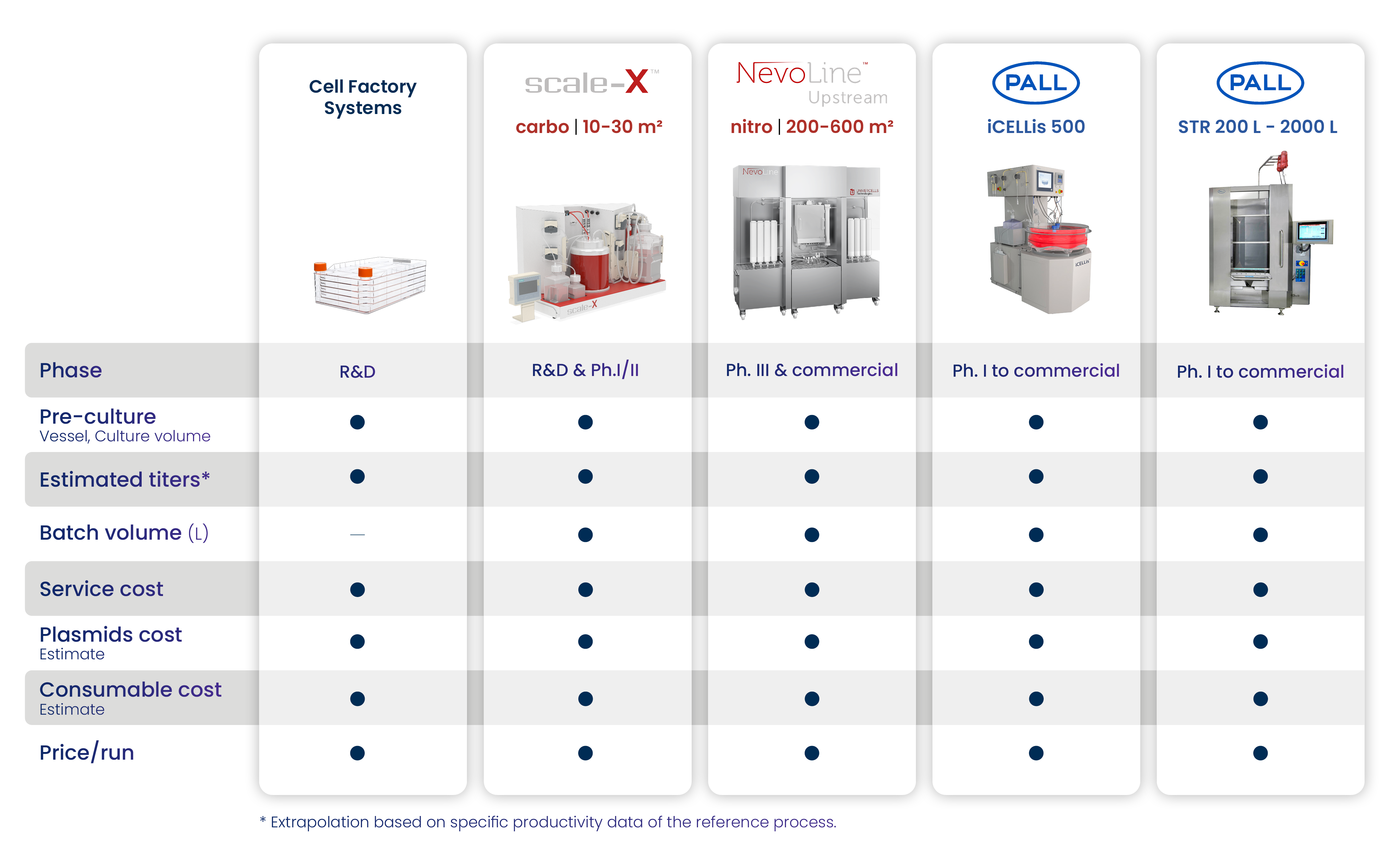
Get a Complete Report
In this complete report, you will receive, among other things, a chart containing all the crucial data relating to the technologies used during the different development phases of your project.
The Methodology
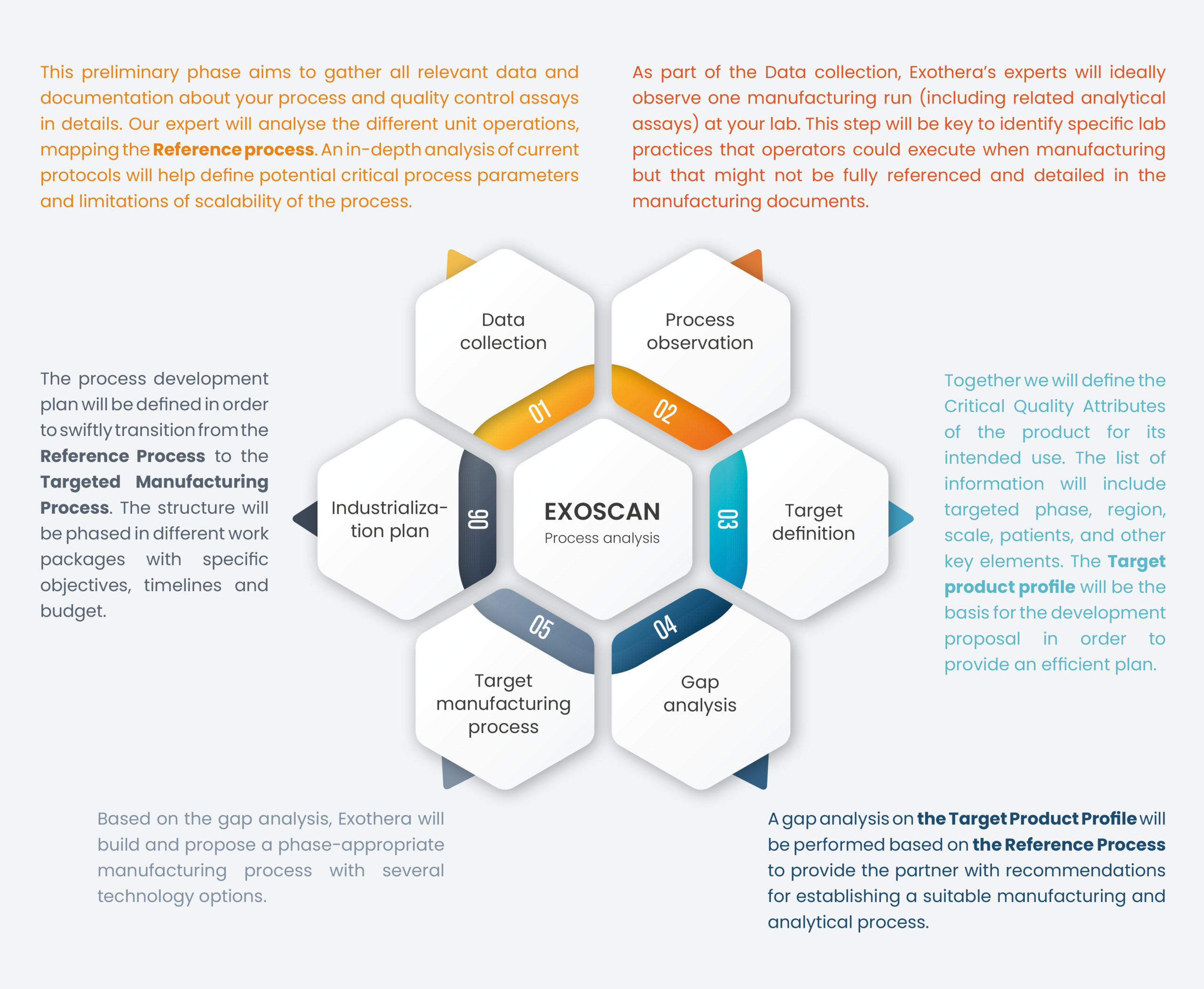
The Methodology
