Ready-to-go viral vector platform
The exoReady™ viral vector platform integrates our high-performing starting materials (cell lines and vector systems) with our data-driven expertise and comprehensive documentation package, including standard operating procedures, bills of testing, and material specifications. This flexible manufacturing technology, combined with our CMC package, offers a complete solution to support the production of viral vector-based vaccines, oncolytic therapies, and gene therapy products

Capitalize on exoREADY technology platform
Viral vector
dedicated platform
- EProprietary manufacturing platform
- EDedicated to industrial manufacturing of viral vectors for Cell and Gene Therapy & Vaccine
- EFully customizable
Ready to use
technology
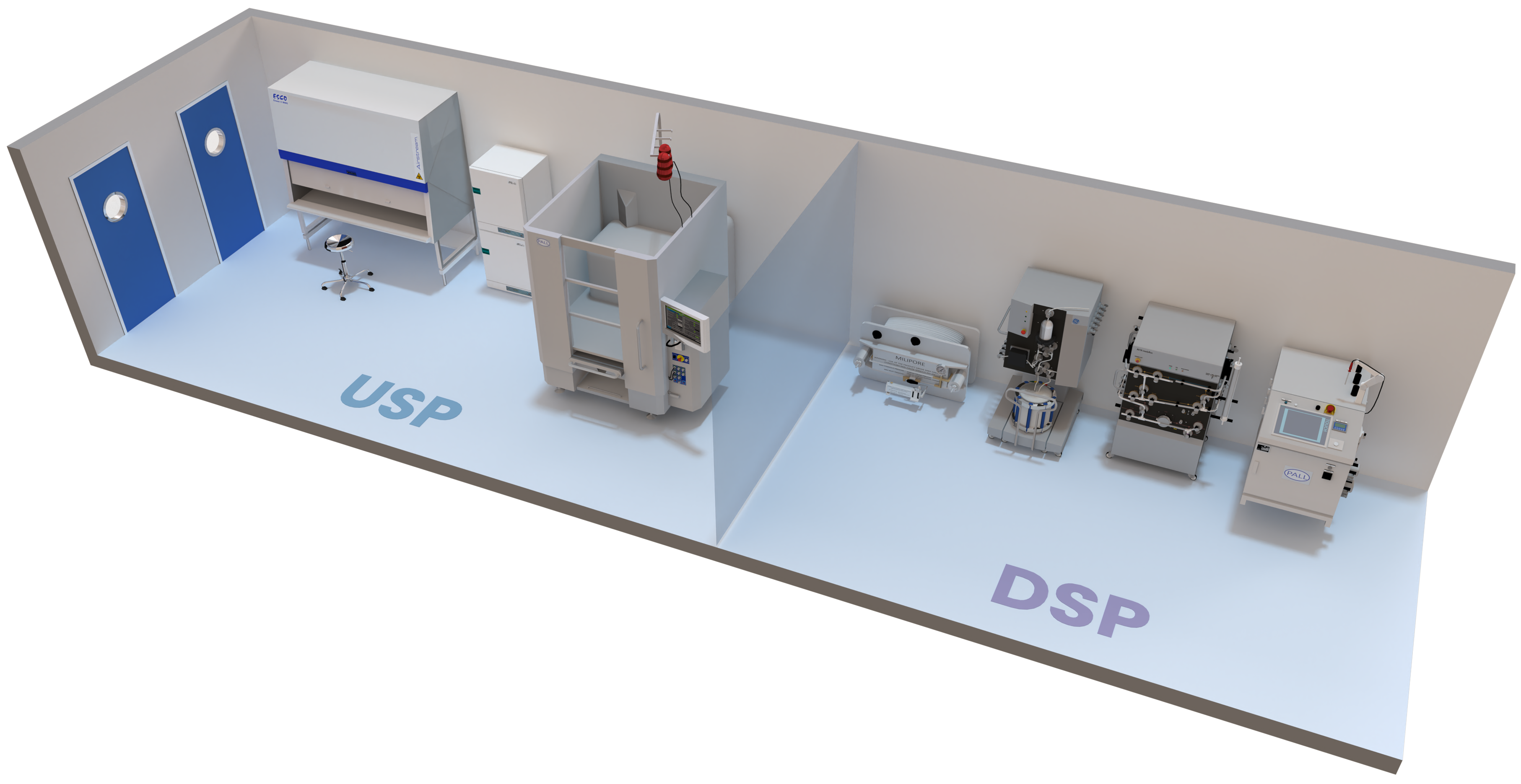
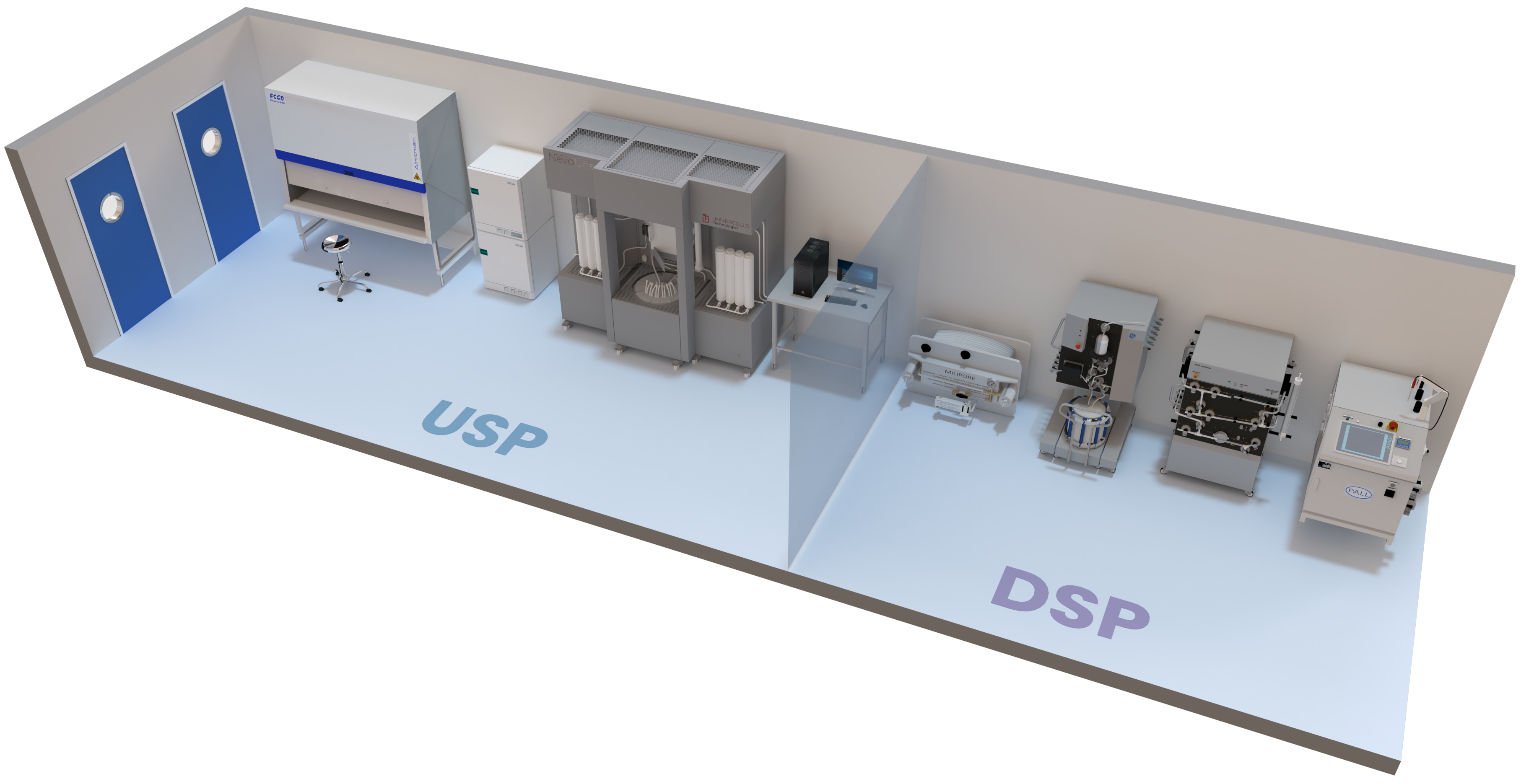
- EState-of-the-art USP equipment
- EState-of-the-art DSP equipment
- EPre-developed analytical toolkit
- ECMC and regulatory support
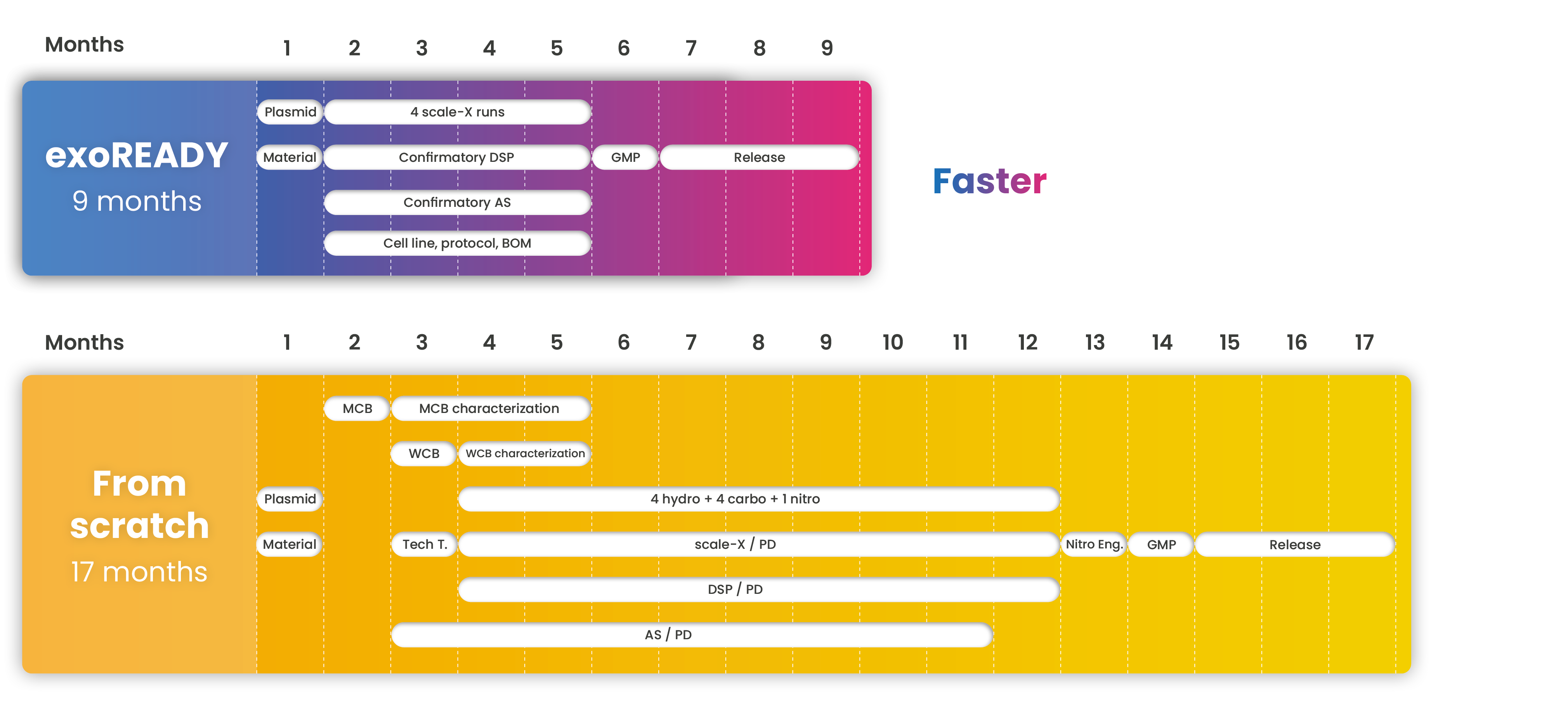
Get faster to the clinical phase without quality compromise
We work day by day to reduce the development timeline without compromising on quality and costs.

Get faster to the clinical phase without quality compromise
We work day by day to reduce the development timeline without compromising on quality and costs.